Induction of CD73 prevents death after emergency open aortic surgery for a ruptured abdominal aortic aneurysm: a randomized, double-blind, placebo-controlled study
- PMID: 35115574
- PMCID: PMC8813993
- DOI: 10.1038/s41598-022-05771-1
Induction of CD73 prevents death after emergency open aortic surgery for a ruptured abdominal aortic aneurysm: a randomized, double-blind, placebo-controlled study
Abstract
Mortality remains high after emergency open surgery for a ruptured abdominal aortic aneurysm (RAAA). The aim of the present study was to assess, if intravenous (IV) Interferon (IFN) beta-1a improve survival after surgery by up-regulating Cluster of differentiation (CD73). This is a multi-center phase II double-blind, 2:1 randomized, parallel group comparison of the efficacy and safety of IV IFN beta-1a vs. placebo for the prevention of death after open surgery for an infra-renal RAAA. All study patients presented a confirmed infra-renal RAAA, survived the primary emergency surgery and were treated with IFN beta-1a (10 μg) or matching placebo for 6 days after surgery. Major exclusion criteria included fatal hemorrhagic shock, chronic renal replacement therapy, diagnosed liver cirrhosis, severe congestive heart failure, advanced malignant disease, primary attempt of endovascular aortic repair (EVAR), and per-operative suprarenal clamping over 30 min. Main outcome measure was all-cause mortality at day 30 (D30) from initial emergency aortic reconstruction. The study was pre-maturely stopped due to a reported drug-drug interaction and was left under-powered. Out of 40 randomized patients 38 were included in the outcome analyses (27 IFN beta-1a and 11 placebo). There was no statistically significant difference between treatment groups at baseline except more open-abdomen and intestinal ischemia was present in the IFN beta-1a arm. D30 all-cause mortality was 22.2% (6/27) in the IFN beta-1a arm and 18.2% (2/11) in the placebo arm (OR 1.30; 95% CI 0.21-8.19). The most common adverse event relating to the IFN beta-1a was pyrexia (20.7% in the IFN beta-1a arm vs. 9.1% in the placebo arm). Patients with high level of serum CD73 associated with survival (P = 0.001) whereas the use of glucocorticoids and the presence of IFN beta-1a neutralizing antibodies associated with a poor CD73 response and survival. The initial aim of the trial, if postoperative INF beta-1a treatment results on better RAAA survival, could not be demonstrated. Nonetheless the anticipated target mechanism up-regulation of CD73 was associated with 100% survival. According to present results the INF beta-1a induced up-regulation of serum CD73 was blocked with both use of glucocorticoids and serum IFN beta-1a neutralizing antibodies. The study was pre-maturely stopped due to interim analysis after a study concerning the use if IV IFN beta-1a in ARDS suggested that the concomitant use of glucocorticoids and IFN beta-1a block the CD73 induction. Trial registration: ClinicalTrials.gov NCT03119701. Registered 19/04/2017 (retrospectively registered).
© 2022. The Author(s).
Conflict of interest statement
Dr Jalkanen and Karvonen own stock and are employed by Faron Pharmaceuticals Ltd. Other authors declare no competing interests.
Figures
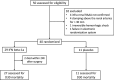


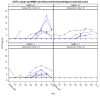
References
-
- van Beek SC, Conijn AP, Koelemay MJ, Balm R. Editor's choice—Endovascular aneurysm repair versus open repair for patients with a ruptured abdominal aortic aneurysm: A systematic review and meta-analysis of short-term survival. Eur. J. Vasc. Endovasc. Surg. 2014;47:593–602. doi: 10.1016/j.ejvs.2014.03.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

