Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial
- PMID: 35115706
- PMCID: PMC10588819
- DOI: 10.1038/s41591-021-01660-8
Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial
Abstract
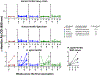
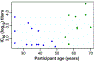
Currently, licensed seasonal influenza vaccines display variable vaccine effectiveness, and there remains a need for novel vaccine platforms capable of inducing broader responses against viral protein domains conserved among influenza subtypes. We conducted a first-in-human, randomized, open-label, phase 1 clinical trial ( NCT03186781 ) to evaluate a novel ferritin (H2HA-Ferritin) nanoparticle influenza vaccine platform. The H2 subtype has not circulated in humans since 1968. Adults born after 1968 have been exposed to only the H1 subtype of group 1 influenza viruses, which shares a conserved stem with H2. Including both H2-naive and H2-exposed adults in the trial allowed us to evaluate memory responses against the conserved stem domain in the presence or absence of pre-existing responses against the immunodominant HA head domain. Fifty healthy participants 18-70 years of age received H2HA-Ferritin intramuscularly as a single 20-μg dose (n = 5) or a 60-μg dose either twice in a homologous (n = 25) prime-boost regimen or once in a heterologous (n = 20) prime-boost regimen after a matched H2 DNA vaccine prime. The primary objective of this trial was to evaluate the safety and tolerability of H2HA-Ferritin either alone or in prime-boost regimens. The secondary objective was to evaluate antibody responses after vaccination. Both vaccines were safe and well tolerated, with the most common solicited symptom being mild headache after both H2HA-Ferritin (n = 15, 22%) and H2 DNA (n = 5, 25%). Exploratory analyses identified neutralizing antibody responses elicited by the H2HA-Ferritin vaccine in both H2-naive and H2-exposed populations. Furthermore, broadly neutralizing antibody responses against group 1 influenza viruses, including both seasonal H1 and avian H5 subtypes, were induced in the H2-naive population through targeting the HA stem. This ferritin nanoparticle vaccine technology represents a novel, safe and immunogenic platform with potential application for pandemic preparedness and universal influenza vaccine development.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Competing interests
The authors declare the following competing interests: M.K., B.S.G. and J.R.M. are named inventors of US patent 9,441,019, titled Influenza Hemagglutinin Protein-based Vaccines, and of several pending applications on related technologies filed by the Department of Health and Human Services (National Institutes of Health). The remaining authors declare no competing interests.
Figures



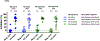
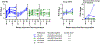





References
-
- World Health Organization. Seasonal influenza. http://www.euro.who.int/en/health-topics/communicable-diseases/influenza... (2021).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

