Comparison of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer After Docetaxel Failure: A Systematic Review and Network Meta-analysis
- PMID: 35115934
- PMCID: PMC8804311
- DOI: 10.3389/fphar.2021.789319
Comparison of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer After Docetaxel Failure: A Systematic Review and Network Meta-analysis
Abstract
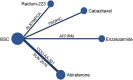
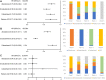
Background: Lacking head-to-head trial, the optimal treatment for patients with metastatic castration-resistant prostate cancer (mCRPC) after docetaxel failure is unclear. This study is to compare the efficacy and safety of systemic treatments in patients who progressed after docetaxel to aid clinical decision-making. Methods: Databases including MEDLINE, EMBASE, and the Cochrane Library were searched from inception to June 15th, 2021. The outcomes of interest include overall survival (OS), biochemical progression-free survival (bPFS), and serious adverse events (SAEs). The Cochrane risk of bias tools were used to assess study quality. Indirect comparisons of competing treatments were performed via Bayesian network meta-analysis. Results: Five trials with 3,862 patients comparing four treatments (abiraterone, enzalutamide, cabazitaxel, and radium-223) were identified. All the four treatments were associated with improved OS and bPFS relative to best supportive care. Among them, enzalutamide (hazard ratio [HR] = 0.58, 95% credible interval [Crl]: 0.49-0.69) had the highest probability of ranking first in terms of OS, followed by cabazitaxel (HR = 0.70, 95% Crl: 0.59-0.83), radium-223 (HR = 0.71, 95% Crl: 0.56-0.90) and abiraterone (HR = 0.73, 95% Crl: 0.63-0.84). Similarly, enzalutamide (HR = 0.25, 95% Crl: 0.20-0.31) showed the greatest improvement of bPFS, followed by abiraterone (HR = 0.60, 95% Crl: 0.51-0.71) and cabazitaxel (HR = 0.75, 95% Crl: 0.63-0.89). In terms of safety, treatments ranked from the safest to the least safe were radium-223 (OR = 0.58, 95% Crl: 0.20-1.68), enzalutamide (OR = 0.80, 95% Crl: 0.28-2.29), abiraterone (OR = 0.94, 95% Crl: 0.39-2.27) and cabazitaxel (OR = 2.50, 95% Crl: 0.84-7.44). Conclusion: For patients with mCRPC who progressed after docetaxel, enzalutamide may offer the most significant survival benefits and satisfying safety. Cabazitaxel is effective in post-docetaxel settings but associated with a high risk of SAEs. Although network meta-analysis provides indirect comparisons and ranking probabilities, the results should be treated with caution as it cannot replace randomized direct comparison. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020223040, identifier CRD42020223040.
Keywords: abirateron; docetaxel (DOC); enzalutamide (ENZ); metastatic castration-resistant prostate cancer; network meta-analysis; radium-223 (Ra) 223.
Copyright © 2022 Chen, Zhang, Zhang, Zhao, Ni, Zhu, He, Dai, Wang, Wang, Liang, Zhu, Shen, Zeng and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Armstrong A. J., Halabi S., Luo J., Nanus D. M., Giannakakou P., Szmulewitz R. Z., et al. (2019). Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J. Clin. Oncol. 37 (13), 1120–1129. 10.1200/JCO.18.01731 - DOI - PMC - PubMed
-
- Bruland Ø. S., Nilsson S., Fisher D. R., Larsen R. H. (2006). High-linear Energy Transfer Irradiation Targeted to Skeletal Metastases by the Alpha-Emitter 223Ra: Adjuvant or Alternative to Conventional Modalities? Clin. Cancer Res. 12 (20 Pt 2), 6250s–6257s. 10.1158/1078-0432.CCR-06-0841 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

