An overview of chemo- and site-selectivity aspects in the chemical conjugation of proteins
- PMID: 35116160
- PMCID: PMC8790347
- DOI: 10.1098/rsos.211563
An overview of chemo- and site-selectivity aspects in the chemical conjugation of proteins
Abstract
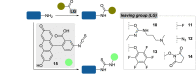
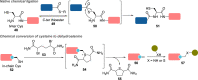
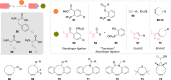
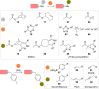
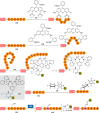
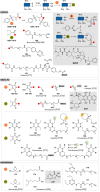
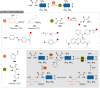
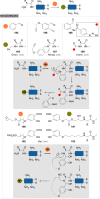
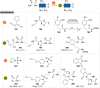
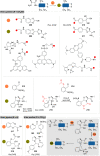
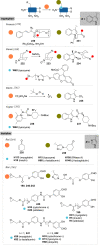
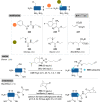
The bioconjugation of proteins-that is, the creation of a covalent link between a protein and any other molecule-has been studied for decades, partly because of the numerous applications of protein conjugates, but also due to the technical challenge it represents. Indeed, proteins possess inner physico-chemical properties-they are sensitive and polynucleophilic macromolecules-that make them complex substrates in conjugation reactions. This complexity arises from the mild conditions imposed by their sensitivity but also from selectivity issues, viz the precise control of the conjugation site on the protein. After decades of research, strategies and reagents have been developed to address two aspects of this selectivity: chemoselectivity-harnessing the reacting chemical functionality-and site-selectivity-controlling the reacting amino acid residue-most notably thanks to the participation of synthetic chemistry in this effort. This review offers an overview of these chemical bioconjugation strategies, insisting on those employing native proteins as substrates, and shows that the field is active and exciting, especially for synthetic chemists seeking new challenges.
Keywords: bioconjugation; chemoselectivity; proteins; site-selectivity; synthetic chemistry.
© 2022 The Authors.
Conflict of interest statement
We have no competing interests.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources
