Combinatorial sympathetic and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockades inhibit the murine melanoma growth by targeting infiltrating T cells
- PMID: 35116419
- PMCID: PMC8798308
- DOI: 10.21037/tcr-20-2738
Combinatorial sympathetic and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockades inhibit the murine melanoma growth by targeting infiltrating T cells
Abstract
Background: Failure of the proliferation and infiltration of tumor-specific T cells in tumor site has been considered as one of important reasons induce the inefficiencies of immune checkpoint therapies in advanced cancers. Therefore, we aimed to demonstrate how combinatorial sympathetic and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade affects the tumor growth of melanoma-bearing mice and potential mechanisms.
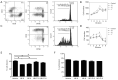
Methods: Tumor growth was measured and the infiltrating immune cell populations were observed with flow cytometry in B16-F10 melanoma-bearing mice treated with combined sympathetic and immune checkpoint blockade, using anti-CTLA-4 antibodies. The expression of adrenergic receptors was investigated in human peripheral blood mononuclear cells and their subpopulations, and the proliferation of T cell subsets was detected when stimulated by norepinephrine and its antagonists.
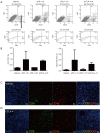
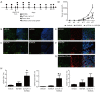
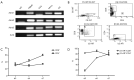
Results: B16-F10 tumor growth was associated with infiltrating CD8+ T cells. Combinatorial sympathetic and CTLA-4 blockade inhibited tumor growth and enhanced CD8+ infiltration. Meanwhile, all β1, β2 and β3 adrenergic receptors were found to be expressed in human peripheral blood mononuclear cells, activated T cells, monocytes, and monocyte-induced dendritic cells. β2-adrenergic receptors were expressed in most CD4+ T cells with increased expression in activated CD8+ T cells. Moreover, norepinephrine was able to prevent CD4+ T cell proliferation and β2-adrenergic receptor antagonists could reverse the inhibition of CD4+, but not CD8+ cell proliferation.
Conclusions: We conclude that the combination of sympathetic and CTLA-4 inhibitors is more effective for inhibiting melanoma progression than a single treatment and might enhance the infiltration of T cells in the tumor site, offering a novel therapeutic approach for immune checkpoint targeting.
Keywords: Sympathetic nerve system; T cells; cytotoxic T-lymphocyte-associated protein 4 inhibitor (CTLA-4 inhibitor); melanoma; β2-adrenergic receptor.
2021 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2738). Dr. MJ reports personal fees from Astrazeneca Japan, outside the submitted work. The other authors have no conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
