MiR-185-3p regulates epithelial mesenchymal transition via PI3K/Akt signaling pathway by targeting cathepsin D in gastric cancer cells
- PMID: 35117305
- PMCID: PMC8799188
- DOI: 10.21037/tcr-19-2133
MiR-185-3p regulates epithelial mesenchymal transition via PI3K/Akt signaling pathway by targeting cathepsin D in gastric cancer cells
Abstract
Background: Recently research reported that miR-185-3p could serve as an independent prognosis factor in gastric cancer (GC). However, the functional role and underlying mechanism of miR-185-3p in GC and epithelial-mesenchymal transition (EMT) progression remains largely elusive.
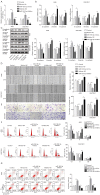
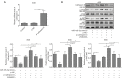
Methods: Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out to analyze the expression of miR-185-3p and cathepsin D in patient-derived GC samples and various GC cell lines. Scratch assay and Transwell assay were used to evaluate the migration ability. The influence of miR-185-3p on the cell cycle distribution and cell apoptosis was evaluated using flow cytometry. Western blotting assay was performed to detect the expression of EMT associated proteins and the activity of PI3K/Akt signaling pathway. Furthermore, the interaction between miR-185-3p and cathepsin D was explored by dual-luciferase reporter assay.
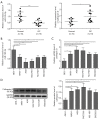
Results: Our data revealed that miR-185-3p was down-regulated, while cathepsin D was up-regulated in both patient-derived GC samples and GC cells. Apart from inducing apoptosis, overexpression of miR-185-3p also inhibited EMT process and migration of GC cells. Mechanically, we firstly verified that miR-185-3p directly targeted the cathepsin D. Furthermore, miR-185-3p exerted its function on EMT process and migration via inhibiting cathepsin D to mediated PI3K/Akt signaling pathway.
Conclusions: Our findings suggested that miR-185-3p targeted cathepsin D inhibiting EMT process via PI3K/Akt signaling, which may serve as a potential prognosis factor and therapeutic target to reduce the malignancy of GCs.
Keywords: MiR-185-3p; PI3K/Akt signaling pathway; epithelial-mesenchymal transition (EMT); gastric cancer (GC).
2020 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/tcr-19-2133). The authors have no conflicts of interest to declare.
Figures


Similar articles
-
MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer.Cancer Biomark. 2019;24(2):159-172. doi: 10.3233/CBM-182000. Cancer Biomark. 2019. PMID: 30614803
-
Targeting of SPP1 by microRNA-340 inhibits gastric cancer cell epithelial-mesenchymal transition through inhibition of the PI3K/AKT signaling pathway.J Cell Physiol. 2019 Aug;234(10):18587-18601. doi: 10.1002/jcp.28497. Epub 2019 Apr 5. J Cell Physiol. 2019. Retraction in: J Cell Physiol. 2022 Mar;237(3):2010. doi: 10.1002/jcp.30522. PMID: 30953349 Retracted.
-
Ropivacaine Inhibits the Growth, Migration and Invasion of Gastric Cancer Through Attenuation of WEE1 and PI3K/AKT Signaling via miR-520a-3p.Onco Targets Ther. 2020 Jun 9;13:5309-5321. doi: 10.2147/OTT.S244550. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606749 Free PMC article.
-
Circular RNA circ_0006089 promotes the progression of gastric cancer by regulating the miR-143-3p/PTBP3 axis and PI3K/AKT signaling pathway.J Dig Dis. 2022 Jul;23(7):376-387. doi: 10.1111/1751-2980.13116. Epub 2022 Aug 23. J Dig Dis. 2022. PMID: 35844201
-
Effects of miR-330-3p on Invasion, Migration and EMT of Gastric Cancer Cells by Targeting PRRX1-Mediated Wnt/β-Catenin Signaling Pathway.Onco Targets Ther. 2020 Apr 22;13:3411-3423. doi: 10.2147/OTT.S238665. eCollection 2020. Onco Targets Ther. 2020. Retraction in: Onco Targets Ther. 2024 Apr 05;17:299-300. doi: 10.2147/OTT.S472066. PMID: 32368097 Free PMC article. Retracted.
Cited by
-
The Roles of microRNA miR-185 in Digestive Tract Cancers.Noncoding RNA. 2022 Oct 8;8(5):67. doi: 10.3390/ncrna8050067. Noncoding RNA. 2022. PMID: 36287119 Free PMC article. Review.
-
Differential Competitive Growth of Transgenic Subclones of Neuroblastoma Cells Expressing Different Levels of Cathepsin D Co-Cultured in 2D and 3D in Response to EGF: Implications in Tumor Heterogeneity and Metastasis.Cancers (Basel). 2024 Mar 29;16(7):1343. doi: 10.3390/cancers16071343. Cancers (Basel). 2024. PMID: 38611021 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
