Tailoring neoadjuvant chemotherapy for patients with breast cancer who have achieved pathologic complete response
- PMID: 35117465
- PMCID: PMC8798334
- DOI: 10.21037/tcr.2020.01.01
Tailoring neoadjuvant chemotherapy for patients with breast cancer who have achieved pathologic complete response
Abstract
Background: We retrospectively examined whether different cycles of chemotherapy affected the prognosis of patients who achieved a pathologic complete response (pCR).
Methods: We reviewed data from patients who achieved pCR after neoadjuvant chemotherapy (NACT) between 2008 and 2018. In total, 286 patients were divided into three groups: group one (n=148, 52%) completed standard chemotherapy cycles before surgery, group two (n=81, 28%) did not complete standard chemotherapy cycles before surgery or received chemotherapy after surgery, and group three (n=57, 20%) did not complete standard chemotherapy cycles before surgery but completed them after surgery. Recurrence-free survival (RFS) was estimated using the Kaplan-Meier method, and differences between groups were evaluated by the log-rank test. Cox proportional hazards regression analysis was adjusted for different NACT groups, age, Ki-67 levels, and clinical stages.
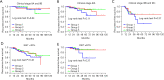
Results: After a median follow-up of 26 months, there were no significant differences in RFS among the NACT groups (P=0.14). Multivariate analysis showed that Ki-67 ≥40% (P=0.03) and clinical stage (IIIB + IIIC) (P=0.002) might be risk factors for recurrence in patients with pCR. There were no significant differences in survival among subgroups according to Ki-67 levels and clinical stages.
Conclusions: Our study suggests that, even with pCR, patients with baseline stage IIIB or IIIC or Ki-67 levels ≥40% may have an increased risk of recurrence. The RFS of patients with pCR was not associated with the completion of standard chemotherapy cycles, even in high-risk patients. Therefore, the prevention of excessive chemotherapeutic treatment by de-escalation is necessary for patients with pCR.
Keywords: Breast cancer; de-escalation of chemotherapy; neoadjuvant chemotherapy (NACT); survival after pathologic complete response (survival after pCR).
2020 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.01). The authors have no conflicts of interest to declare.
Figures


References
-
- Winer EP. Adjuvant and neoadjuvant therapy in patients with early breast cancer: Principles and practical considerations. The Breast 2019. doi: .10.1016/S0960-9776(19)30093-1 - DOI
LinkOut - more resources
Full Text Sources
