SARS-COV-2 Variants: Differences and Potential of Immune Evasion
- PMID: 35118007
- PMCID: PMC8805732
- DOI: 10.3389/fcimb.2021.781429
SARS-COV-2 Variants: Differences and Potential of Immune Evasion
Abstract
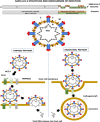
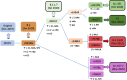
The structural spike (S) glycoprotein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) plays an essential role in infection and is an important target for neutralizing antibody recognition. Mutations in the S gene can generate variants of concern (VOCs), which improve "viral fitness" through selective or survival advantages, such as increased ACE-2 receptor affinity, infectivity, viral replication, higher transmissibility, resistance to neutralizing antibodies and immune escape, increasing disease severity and reinfection risk. Five VOCs have been recognized and include B.1.1.7 (U.K.), B.1.351 (South Africa), P.1 (Brazil), B.1.617.2 (India), and B.1.1.529 (multiple countries). In this review, we addressed the following critical points concerning VOCs: a) characteristics of the SARS-CoV-2 VOCs with mutations in the S gene; b) possible evasion of variants from neutralizing antibodies generated through vaccination, previous infection, or immune therapies; c) potential risk of new pandemic waves induced by the variants worldwide; and d) perspectives for further studies and actions aimed at preventing or reducing the impact of new variants during the current COVID-19 pandemic.
Keywords: COVID-19; delta variant; immune escape; neutralizing antibody; omicron variant; vaccines; variant of concern.
Copyright © 2022 Hirabara, Serdan, Gorjao, Masi, Pithon-Curi, Covas, Curi and Durigon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

