Study Protocol of the Exercise Study: Unraveling Limitations for Physical Activity in Children With Chronic Diseases in Order to Target Them With Tailored Interventions-A Randomized Cross Over Trial
- PMID: 35118031
- PMCID: PMC8805206
- DOI: 10.3389/fped.2021.791701
Study Protocol of the Exercise Study: Unraveling Limitations for Physical Activity in Children With Chronic Diseases in Order to Target Them With Tailored Interventions-A Randomized Cross Over Trial
Abstract
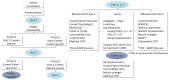
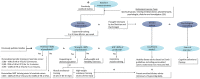
Introduction: Physical activity is associated with many physiological and psychological health benefits across the lifespan. Children with a chronic disease often have lower levels of daily physical activity, and a decreased exercise capacity compared to healthy peers. In order to learn more about limitations for physical activity, we investigate children with four different chronic diseases: children with a Fontan circulation, children with Broncho Pulmonary Dysplasia (BPD), Pompe disease and inflammatory bowel disease (IBD). Each of these diseases is likely to interfere with physical activity in a different way. Knowing the specific limitations for physical activity would make it possible to target these, and increase physical activity by a personalized intervention. The aim of this study is to first investigate limitations for physical activity in children with various chronic diseases. Secondly, to measure the effects of a tailored exercise intervention, possibly including a personalized dietary advice and/or psychological counseling, on exercise capacity, endurance, quality of life, fatigue, fear for exercise, safety, muscle strength, physical activity levels, energy balance, and body composition. Methods and Analysis: This randomized crossover trial will aim to include 72 children, aged 6-18 years, with one of the following diagnosis: a Fontan circulation, BPD, Pompe disease and IBD. Eligible patients will participate in the 12-week tailored exercise intervention and are either randomized to start with a control period or start with the intervention. The tailored 12-week exercise interventions, possibly including a personalized dietary advice and/or psychological counseling, will be designed based on the found limitations for physical activity in each disease group during baseline measurements by the Rotterdam Exercise Team. Effects of the tailored training interventions will be measured on the following endpoints: exercise capacity (measured by cardiopulmonary exercise test), endurance, physical activity levels, muscle strength, quality of life, fatigue, fear for exercise, disease activity, cardiac function (in children with a Fontan circulation), energy balance, and body composition. Ethics and Dissemination: Conducted according to the Declaration of Helsinki and Good Clinical Practice. Medical-ethical approval was obtained. Trial Registration Number: NL8181, https://www.trialregister.nl/trial/8181.
Keywords: Broncho Pulmonary Dysplasia; Fontan—total cavopulmonary; Pompe disease; inflammatory bowel disease; lifestyle intervention; pediatrics; physical activity; physical exercise.
Copyright © 2022 Scheffers, Helbing, Utens, Dieleman, Dulfer, Noske, van den Broek, Walet, Olieman, Escher, Pijnenburg, van der Ploeg and van den Berg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

