Cryogenic 3D Printing of ß-TCP/PLGA Composite Scaffolds Incorporated With BpV (Pic) for Treating Early Avascular Necrosis of Femoral Head
- PMID: 35118053
- PMCID: PMC8804314
- DOI: 10.3389/fbioe.2021.748151
Cryogenic 3D Printing of ß-TCP/PLGA Composite Scaffolds Incorporated With BpV (Pic) for Treating Early Avascular Necrosis of Femoral Head
Abstract
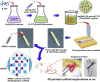
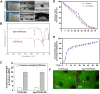
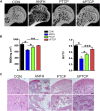
Avascular necrosis of femoral head (ANFH) is a disease that is characterized by structural changes and collapse of the femoral head. The exact causes of ANFH are not yet clear, but small advances in etiopathogenesis, diagnosis and treatment are achieved. In this study, ß-tricalcium phosphate/poly lactic-co-glycolic acid composite scaffolds incorporated with bisperoxovanadium [bpV (pic)] (bPTCP) was fabricated through cryogenic 3D printing and were utilized to treat rat models with early ANFH, which were constructed by alcohol gavage for 6 months. The physical properties of bPTCP scaffolds and in vitro bpV (pic) release from the scaffolds were assessed. It was found that the sustained release of bpV (pic) promoted osteogenic differentiation and inhibited adipose differentiation of bone marrow-derived mesenchymal stem cells. Micro-computed tomography scanning and histological analysis confirmed that the progression of ANFH in rats was notably alleviated in bPTCP scaffolds. Moreover, it was noted that the bPTCP scaffolds inhibited phosphatase and tensin homolog and activated the mechanistic target of rapamycin signaling. The autophagy induced by bPTCP scaffolds could partially prevent apoptosis, promote osteogenesis and angiogenesis, and hence eventually prevent the progression of ANFH, suggesting that the bPTCP scaffold are promising candidate to treat ANFH.
Keywords: avascular necrosis of femoral head; bisperoxovanadium; phosphatase and tensin homolo; rats; ß-tricalcium phosphate.
Copyright © 2022 Li, Cao, Li, Huang, Yang, Li, Zheng, Ye, Zhou, Peng, Liu, Wang, Xie, Tang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

