Circulating Microparticles in the Pathogenesis and Early Anticoagulation of Thrombosis in COVID-19 With Kidney Injury
- PMID: 35118071
- PMCID: PMC8804312
- DOI: 10.3389/fcell.2021.784505
Circulating Microparticles in the Pathogenesis and Early Anticoagulation of Thrombosis in COVID-19 With Kidney Injury
Abstract
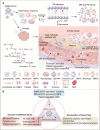
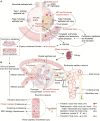
As more is learned about the pathophysiological mechanisms of COVID-19, systemic thrombosis has been recognized as being associated with more severe clinical manifestations, mortality and sequelae. As many as 40% of patients admitted to the hospital due to COVID-19 have acute kidney injury, with coagulation abnormalities the main cause of impaired function. However, the mechanism of renal thrombosis and the process leading to kidney injury are unclear. Microparticles (MPs) are membrane bubbles released in response to activation, injury or apoptosis of cells. The phosphatidylserine (PS) exposed on the surface of MPs provides binding sites for endogenous and exogenous FXase complexes and prothrombin complexes, thus providing a platform for the coagulation cascade reaction and facilitating clot formation. In the context of COVID-19 infection, viral attack leads immune cells to release cytokines that damage circulating blood cells and vascular endothelial cells, resulting in increased MPs levels. Therefore, MPs can be used as a risk factor to predict renal microthrombosis and kidney injury. In this paper, we have summarized the latest data on the pathophysiological mechanism and treatment of renal thrombosis caused by MPs in COVID-19, revealing that the coagulation abnormality caused by MP and PS storms is a universal progression that aggravates the mortality and sequelae of COVID-19 and potentially other pandemic diseases. This paper also describes the risk factors affecting renal thrombosis in COVID-19 from the perspective of the Virchow's triad: blood hypercoagulability, vascular endothelial injury, and decreased blood flow velocity. In summary, given the serious consequences of thrombosis, current guidelines and clinical studies suggest that early prophylactic anticoagulant therapy reduces mortality and improves clinical outcomes. Early anticoagulation, through inhibition of PS-mediated coagulopathy, allows maintenance of unobstructed blood circulation and oxygen delivery thereby facilitating the removal of inflammatory factors, viruses, MPs, and dead or damaged cells, and expediting patient rehabilitation.
Keywords: COVID-19; early anticoagulation; kidney; microparticle; phosphatidylserine; thrombosis.
Copyright © 2022 Wang, Yu, Novakovic, Xie and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pathophysiological mechanisms of thrombosis in acute and long COVID-19.Front Immunol. 2022 Nov 16;13:992384. doi: 10.3389/fimmu.2022.992384. eCollection 2022. Front Immunol. 2022. PMID: 36466841 Free PMC article. Review.
-
The Central Role of Extracellular Vesicles in the Mechanisms of Thrombosis in COVID-19 Patients With Cancer and Therapeutic Strategies.Front Cell Dev Biol. 2022 Jan 12;9:792335. doi: 10.3389/fcell.2021.792335. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35096822 Free PMC article. Review.
-
The cross-talk of lung and heart complications in COVID-19: Endothelial cells dysfunction, thrombosis, and treatment.Front Cardiovasc Med. 2022 Aug 5;9:957006. doi: 10.3389/fcvm.2022.957006. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35990983 Free PMC article. Review.
-
Long COVID: The Nature of Thrombotic Sequelae Determines the Necessity of Early Anticoagulation.Front Cell Infect Microbiol. 2022 Apr 5;12:861703. doi: 10.3389/fcimb.2022.861703. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35449732 Free PMC article. Review.
-
Increased blood cell phosphatidylserine exposure and circulating microparticles contribute to procoagulant activity after carotid artery stenting.J Neurosurg. 2017 Nov;127(5):1041-1054. doi: 10.3171/2016.8.JNS16996. Epub 2016 Dec 23. J Neurosurg. 2017. PMID: 28009236
Cited by
-
Pathophysiological mechanisms of thrombosis in acute and long COVID-19.Front Immunol. 2022 Nov 16;13:992384. doi: 10.3389/fimmu.2022.992384. eCollection 2022. Front Immunol. 2022. PMID: 36466841 Free PMC article. Review.
-
Hematological Markers in Thromboembolic Events: A Comparative Study of COVID-19 and Non-COVID-19 Hospitalized Patients.J Clin Med. 2025 May 5;14(9):3192. doi: 10.3390/jcm14093192. J Clin Med. 2025. PMID: 40364223 Free PMC article.
-
Exploring Urinary Extracellular Vesicles and Immune Mediators as Biomarkers of Kidney Injury in COVID-19 Hospitalized Patients.Diagnostics (Basel). 2022 Oct 27;12(11):2600. doi: 10.3390/diagnostics12112600. Diagnostics (Basel). 2022. PMID: 36359444 Free PMC article.
-
Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19.J Clin Med. 2023 Jan 25;12(3):929. doi: 10.3390/jcm12030929. J Clin Med. 2023. PMID: 36769577 Free PMC article.
-
Real-world practices of low-molecular-weight heparin for venous thromboembolism prophylaxis in patients hospitalized with COVID-19: a multicenter prospective study from China.Thromb J. 2025 Jun 20;23(1):69. doi: 10.1186/s12959-025-00741-9. Thromb J. 2025. PMID: 40542344 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

