Isolation of Mitochondria from Ustilago maydis Protoplasts
- PMID: 35118170
- PMCID: PMC8769755
- DOI: 10.21769/BioProtoc.4277
Isolation of Mitochondria from Ustilago maydis Protoplasts
Abstract
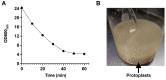
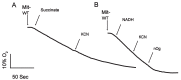
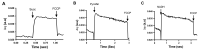
Ustilago maydis, a basidiomycete that infects Zea mays, is one of the top ten fungal models for studying DNA repair, signal transduction pathways, and dimorphic transitions, among other processes. From a metabolic point of view, U. maydis lacks fermentative capacity, pointing to mitochondria as a key player in central metabolism. Oxidative phosphorylation, synthesis of heme groups, Krebs cycle, β-oxidation of fatty acids, and synthesis of amino acids are some of the processes that take place in mitochondria. Given the importance of this organelle in eukaryotic cells in general, and in fungal cells in particular, we present a protocol for the isolation of U. maydis mitochondria based on the enzymatic disruption of U. maydis cell wall and differential centrifugation. The method can easily be extrapolated to other fungal species, by using appropriate lytic enzymes.
Keywords: Differential centrifugation; Lysing cell wall; Membrane potential; Mitochondria isolation; Respiratory chain; Ustilago maydis.
Copyright © 2022 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe author declares no competing interest related to this work.
Figures
References
-
- Akerman K. E. and Wikström M. K.(1976). Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68(2): 191-197. - PubMed
-
- Brefort T., Doehlemann G., Mendoza-Mendoza A., Reissmann S., Djamei A. and Kahmann R.(2009). Ustilago maydis as a Pathogen . Annu Rev Phytopathol 47: 423-445. - PubMed
-
- Figueira T. R., Melo D. R., Vercesi A. E. and Castilho R. F.(2012). Safranine as a fluorescent probe for the evaluation of mitochondrial membrane potential in isolated organelles and permeabilized cells. Methods Mol Biol 810: 103-117. - PubMed
-
- Juárez O., Guerra G., Martínez F. and Pardo J. P.(2004). The mitochondrial respiratory chain of Ustilago maydis . Biochim Biophys Acta 1658(3): 244-251. - PubMed
-
- Lowry O. H., Rosebrough N. J., Farr A. L. and Randall R. J.(1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193(1): 265-275. - PubMed
LinkOut - more resources
Full Text Sources

