Radiation-induced neuroinflammation: a potential protective role for poly(ADP-ribose) polymerase inhibitors?
- PMID: 35118383
- PMCID: PMC8807076
- DOI: 10.1093/noajnl/vdab190
Radiation-induced neuroinflammation: a potential protective role for poly(ADP-ribose) polymerase inhibitors?
Abstract
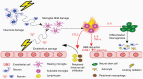
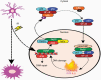
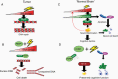
Radiotherapy (RT) plays a fundamental role in the treatment of glioblastoma (GBM). GBM are notoriously invasive and harbor a subpopulation of cells with stem-like features which exhibit upregulation of the DNA damage response (DDR) and are radioresistant. High radiation doses are therefore delivered to large brain volumes and are known to extend survival but also cause delayed toxicity with 50%-90% of patients developing neurocognitive dysfunction. Emerging evidence identifies neuroinflammation as a critical mediator of the adverse effects of RT on cognitive function. In addition to its well-established role in promoting repair of radiation-induced DNA damage, activation of poly(ADP-ribose) polymerase (PARP) can exacerbate neuroinflammation by promoting secretion of inflammatory mediators. Therefore, PARP represents an intriguing mechanistic link between radiation-induced activation of the DDR and subsequent neuroinflammation. PARP inhibitors (PARPi) have emerged as promising new agents for GBM when given in combination with RT, with multiple preclinical studies demonstrating radiosensitizing effects and at least 3 compounds being evaluated in clinical trials. We propose that concomitant use of PARPi could reduce radiation-induced neuroinflammation and reduce the severity of radiation-induced cognitive dysfunction while at the same time improving tumor control by enhancing radiosensitivity.
Keywords: DNA damage; glioblastoma; microglia; neuroinflammation; neuroprotection; radiation therapy.
© The Author(s) 2022. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures
References
-
- Walker MD, Green SB, Byar DP, et al. . Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303(23):1323–1329. - PubMed
-
- Bao S, Wu Q, McLendon RE, et al. . Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. - PubMed
-
- Barazzuol L, Hopkins SR, Ju L, Jeggo PA. Distinct response of adult neural stem cells to low versus high dose ionising radiation. DNA Repair (Amst). 2019;76:70–75. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
