Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression
- PMID: 35118423
- PMCID: PMC8809887
- DOI: 10.1038/s43018-021-00232-6
Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression
Abstract
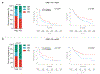
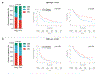
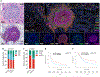
Only a minority of patients derive long-term clinical benefit from anti-PD1/PD-L1 monoclonal antibodies. The presence of tertiary lymphoid structures (TLS) has been associated with improved survival in several tumor types. Here, using a large-scale retrospective analysis of three independent cohorts of cancer patients treated with anti-PD1/PD-L1 antibodies, we showed that the presence of mature TLS was associated with improved objective response rate, progression-free survival, and overall survival independently of PD-L1 expression status and CD8+ T-cell density. These results pave the way for using TLS detection to select patients who are more likely to benefit from immune checkpoint blockade.
Keywords: Immune checkpoint inhibitors; biomarkers; tertiary lymphoid structures (TLS); tumor microenvironment.
Conflict of interest statement
DECLARATION OF INTERESTS LV, MB, SC, SLM, ML, IS, MT, GR, SP, MC, FC, CL, KB, MK, IG, CSF, VV, FC, WHF, and FLL: Nothing to disclose AB, JPG, and CR: Employees of Immusmol/Explicyte EO, AS: Employees of Astra Zeneca AI: Received research grants from Astra Zeneca, Bayer, BMS, Chugai, Merck, MSD, Pharmamar, Novartis, Roche, and received personal fees from Epizyme, Bayer, Lilly, Roche, and Springworks BB: Received grants from AstraZeneca , Pfizer , Eli Lilly , Onxeo , Bristol Myers Squibb , Inivata , Abbvie , Amgen , Blueprint Medicines , Celgene , GlaxoSmithKline , Ignyta , Ipsen , Merck KGaA , MSD Oncology , Nektar , PharmaMar , Sanofi , Spectrum Pharmaceuticals , Takeda , Tiziana Therapeutics , Cristal Therapeutics , Daiichi Sankyo , Janssen Oncology , OSE Immunotherapeutics , BeiGene , Boehringer Ingelheim , Genentech , SERVIER , Tolero Pharmaceuticals YL: Received grants and personal fees from Janssen, during the conduct of the study; personal fees and non-financial support from Astellas, grants and personal fees from Sanofi, personal fees and non-financial support from Roche, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from MSD, personal fees and non-financial support from BMS, personal fees from Clovis , personal fees and non-financial support from Seattle Genetics, personal fees from Incyte, personal fees from Pfizer. AM: Received research grants from Mersu, Bristol-Myers Squibb, Boehringer Ingelheim, Transgene, MSD and received personal fees from Bristol-Myers Squibb, AstraZeneca, MedImmune, Oncovir, Merieux JCS: Has received consultancy fees from AstraZeneca, Astex, Clovis, GSK, GamaMabs, Lilly, MSD, Mission Therapeutics, Merus, Pfizer, Pharma Mar, Pierre Fabre, Roche/Genentech, Sanofi, Servier, Symphogen, and Takeda.
Figures

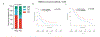


References
-
- Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

