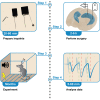
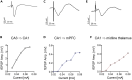
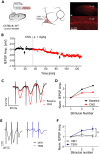
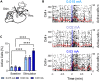
Measuring synaptic transmission and plasticity with fEPSP recordings in behaving mice
- PMID: 35118427
- PMCID: PMC8792427
- DOI: 10.1016/j.xpro.2021.101115
Measuring synaptic transmission and plasticity with fEPSP recordings in behaving mice
Abstract
Spontaneous spiking activity depends on intrinsic excitability and synaptic input. Historically, synaptic activity has been mostly studied ex vivo. Here, we describe a versatile and robust protocol to record field excitatory postsynaptic potentials (fEPSPs) in behaving rodents. The protocol allows estimating the input-output relationship of a specific pathway, short-term and long-term plasticity, and their modulation by pharmacological or pharmacogenetic interventions and behavioral states. However, experimenters must be aware of the protocol's specificity and interpret results with care. For complete details on the use and execution of this profile, please refer to Styr et al. (2019).
Keywords: Behavior; Microscopy; Neuroscience.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Chimene M.F., Pallas-Areny R. A comprehensive model for power line interference in biopotential measurements. IEEE Trans. Instrum. Meas. 2000;49:535–540.
-
- Curzon P., Rustay N.R., Browman K.E. In: Methods of Behavior Analysis in Neuroscience. Buccafusco J.J., editor. CRC Press/Taylor & Francis; 2009. Cued and contextual fear conditioning for rodents. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

