Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination
- PMID: 35118442
- PMCID: PMC8320012
- DOI: 10.17925/EE.2021.17.1.12
Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination
Abstract
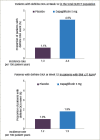
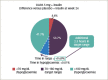
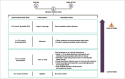
The prevalence of type 1 diabetes (T1D) is increasing worldwide. T1D reduces life expectancy due to complications including cardiovascular disease. Sodium-glucose co-transporter (SGLT) inhibitors are a new class of drugs developed to treat type 2 diabetes (T2D), and now they can be used as an adjunct to insulin in T1D. In clinical trials, they have been shown to improve glycaemic control and decrease body weight without the risk of increased hypoglycaemia and with a reduction in insulin dose. Four SGLT2 inhibitors have been approved in Europe for the treatment of T2D, while only dapagliflozin and sotagliflozin, a dual SGLT1 and SGLT2 inhibitor approved in 2019, have been approved for the treatment of T1D. Both can be used as an adjunct therapy in combination with insulin in adults with a body mass index (BMI) of ≥27 kg/m2, inadequately controlled with insulin. In Europe, dapagliflozin is the only currently available SGLT2 inhibitor indcated as adjunct therapy for patients with T1D. The subgroup of patients with a BMI of ≥27 kg/m2 from the DEPICT-1 and -2 trials (Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 diabetes) showed similar reduction in hyperglycaemia and body weight but no significant increased risk of diabetic ketoacidosis (DKA) than the overall trial population. The risk of DKA has been shown to increase in patients with T1D treated with adjunct therapy with SGLT2 inhibitors, and studies on sotagliflozin and empagliflozin have suggested a dose response. Thus, it is important to educate patients and doctors how to recognize symptoms of upcoming DKA and mitigate it. An independent DKA education programme has recently been developed to instruct patients with T1D being treated with SGLT inhibitor therapies with and without insulin pumps to prevent, identify and treat DKA. Despite these considerations, clinical trials support the use of SGLT2 inhibitors in the management of T1D. The benefits and potential risks of dapagliflozin as an adjunct therapy to insulin in adults with T1D should be considered in each individual case. Here we discuss the efficacy and safety of dapagliflozin as adjunct therapy in patients with T1D.
Keywords: Dapagliflozin; adjunctive therapy; insulin; sodium glucose co-transporter; type 1 diabetes.
© Touch Medical Media 2021.
Conflict of interest statement
Disclosures: Johan H Jendle serves on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic, Merck, NovoNordisk and Sanofi; speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic, NovoNordisk and Sanofi; and has received grants to institution from Medtronic and NovoNordisk. Francisco J Ampudia-Blasco has served on advisory panels for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan, Medtronic, Merck, Novartis, NovoNordisk, Pfizer, Roche and Sanofi; and has received research support from Abbott, AstraZeneca, Boehringer Ingelheim, Bayer, Eli Lilly, GlaxoSmithKline, LifeScan, Merck, NovoNordisk, Pfizer, Sanofi and Servier. Martin Füchtenbusch serves on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Berlin Chemie; and is a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Berlin Chemie, Abbott, Sanofi, MSD and NovoNordisk. Paolo Pozzilli serves on advisory boards for AstraZeneca and Sanofi; speakers bureau for Eli Lilly, Sanofi and AstraZeneca; and has received grants to institution from Sanofi, Eli Lilly, AstraZeneca, Medtronic, GSK, Augen and NovoNordisk.
Figures



References
-
- International Diabetes Federation. IDF Diabetes Atlas 9th edition 2019, 2019.. www.diabetesatlas.org/en/resources/ Available at: (accessed 11 August 2020).
-
- Patterson CC,, Harjutsalo V,, Rosenbauer J,. et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62:409–17. - PubMed
-
- Dandona P,, Mathieu C,, Phillip M,. et al. Efficacy and safety of dapaglifozin in patents with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864–76. - PubMed
-
- Mathieu C,, Dandona P,, Gillard P,. et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41:1938–46. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
