Bleeding complications in patients on new oral anticoagulants for venous thromboembolism in Kenya
- PMID: 35118489
- PMCID: PMC9650143
- DOI: 10.5830/CVJA-2021-060
Bleeding complications in patients on new oral anticoagulants for venous thromboembolism in Kenya
Abstract
Background: The incidence of bleeding complications in patients with venous thromboembolism (VTE) on new oral anticoagulants (NOACs) has not been widely studied in contemporary clinical practice in Africa. The purpose of this study was to determine the rates of major bleeding, clinically relevant non-major bleeding (CRNM) and minor bleeding associated with NOAC use.
Methods: A retrospective review was carried out of patients diagnosed with venous thromboembolism and treated with NOACs at the Aga Khan University Hospital, Nairobi, from January 2014 to December 2019. Clinical and outcome data were collected from medical records and the hospital mortality database. All patients with VTE aged > 18 years and initiated on NOACS were recruited. Patients with missing information were excluded. They were followed up from the time of commencement of oral anticoagulation to completion of therapy, or to the time of the first major bleed, CRNM or minor bleeding. Data on bleeding were obtained from the hospital database and through telephone interviews. Unadjusted rates of the first major bleeding event or CRNM were calculated as the number of bleeding events per 100 person-years.
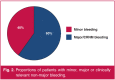
Results: Two hundred and forty-three patients with VTE were recruited and 222 (91.4%) were initiated on rivaroxaban, 12 (4.9%) on dabigatran and nine (3.7%) on apixaban, with a median follow up of 213 [interquartile range (IQR): 119-477] days. The median age of the patients was 57 (IQR: 45-71) years. A total of 64 bleeding events were identified in 50 (20.6%) patients. Overall, the incidence rate for bleeding events was 17.24 per 100 patient-years. The incidence rate of major bleeding was 3.79 per 100 person-years. Gastrointestinal bleeding was the most common major bleeding site. There were more females with bleeding events (70.7%) compared to males. Anaemia and the use of aspirin and other antiplatelets were associated with a higher incidence of major and CRNM bleeding [relative risk (RR) = 3.77, confidence interval (CI) = 1.37-10.39, p = 0.005 and RR = 8.89, CI = 2.06-38.33, p = 0.0003, respectively].
Conclusions: Most of these bleeds were minor, with the gastrointestinal tract being the most common source of major bleeding and menorrhagia being the commonest cause of bleeding. Anaemia and the use of aspirin were associated with a higher incidence of major bleeding.
Keywords: bleeding; deep venous thrombosis (DVT); new oral anticoagulants (NOAC); pulmonary embolism (PE); venous thromboembolism (VTE).
Figures
References
-
- Ageno W, Mantovani LG, Haas S, Kreutz R, Monje D, Schneider J. et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. Lancet Haematol. 2016;3(1):e12–e21. - PubMed
-
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M. et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous