The netrin-1 receptor DCC promotes the survival of a subpopulation of midbrain dopaminergic neurons: Relevance for ageing and Parkinson's disease
- PMID: 35118677
- PMCID: PMC9305203
- DOI: 10.1111/jnc.15579
The netrin-1 receptor DCC promotes the survival of a subpopulation of midbrain dopaminergic neurons: Relevance for ageing and Parkinson's disease
Abstract
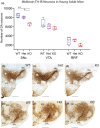
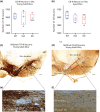
Mechanisms that determine the survival of midbrain dopaminergic (mDA) neurons in the adult central nervous system (CNS) are not fully understood. Netrins are a family of secreted proteins that are essential for normal neural development. In the mature CNS, mDA neurons express particularly high levels of netrin-1 and its receptor Deleted in Colorectal Cancer (DCC). Recent findings indicate that overexpressing netrin-1 protects mDA neurons in animal models of Parkinson's disease (PD), with a proposed pro-apoptotic dependence function for DCC that triggers cell death in the absence of a ligand. Here, we sought to determine if DCC expression influences mDA neuron survival in young adult and ageing mice. To circumvent the perinatal lethality of DCC null mice, we selectively deleted DCC from mDA neurons utilizing DATcre /loxP gene-targeting and examined neuronal survival in adult and aged animals. Reduced numbers of mDA neurons were detected in the substantia nigra pars compacta (SNc) of young adult DATcre /DCCfl/fl mice, with further reduction in aged DATcre /DCCfl/fl animals. In contrast to young adults, aged mice also exhibited a gene dosage effect, with fewer SNc mDA neurons in DCC heterozygotes (DATcre /DCCfl/wt ). Notably, loss of mDA neurons in the SN was not uniform. Neuronal loss in the SN was limited to ventral tier mDA neurons, while mDA neurons in the dorsal tier of the SN, which resist degeneration in PD, were spared from the effect of DCC deletion in both young and aged mice. In the ventral tegmental area (VTA), young adult mice with conditional deletion of DCC had normal mDA neuronal numbers, while significant loss occurred in aged DATcre /DCCfl/fl and DATcre /DCCfl/wt mice compared to age-matched wild-type mice. Our results indicate that expression of DCC is required for the survival of subpopulations of mDA neurons and may be relevant to the degenerative processes in PD.
Keywords: Parkinson’s Disease; calbindin D-28k; dopamine; netrin-1; substantia nigra; unbiased stereology; ventral tegmental area.
© 2022 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflicts to report.
Figures


Comment in
-
The role of DCC in the survival of discrete midbrain dopaminergic neuron subpopulations.J Neurochem. 2022 May;161(3):217-218. doi: 10.1111/jnc.15580. Epub 2022 Feb 7. J Neurochem. 2022. PMID: 35129216 No abstract available.
References
-
- Airavaara, M. , Parkkinen, I. , Konovalova, J. , Albert, K. , Chmielarz, P. , & Domanskyi, A. (2020). Back and to the Future: From Neurotoxin‐Induced to Human Parkinson's Disease Models. Current Protocols in Neuroscience, 91, e88. - PubMed
-
- Backman, C. M. , Malik, N. , Zhang, Y. , Shan, L. , Grinberg, A. , Hoffer, B. J. , Westphal, H. , & Tomac, A. C. (2006). Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis, 44, 383–390. - PubMed
-
- Bayer, S. A. , Wills, K. V. , Triarhou, L. C. , & Ghetti, B. (1995). Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Experimental Brain Research, 105, 191–199. - PubMed
-
- Bin, J. M. , Han, D. , Sun, K. L. W. , Croteau, L. P. , Dumontier, E. , Cloutier, J. F. , Kania, A. , & Kennedy, T. E. (2015). Complete Loss of Netrin‐1 Results in Embryonic Lethality and Severe Axon Guidance Defects without Increased Neural Cell Death. Cell Reports, 12, 1099–1106. - PubMed
-
- Brignani, S. , Raj, D. D. A. , Schmidt, E. R. E. , Düdükcü, Ö. , Adolfs, Y. , De Ruiter, A. A. , Rybiczka‐Tesulov, M. , Verhagen, M. G. , van der Meer, C. , Broekhoven, M. H. , & Moreno‐Bravo, J. A. (2020). Remotely Produced and Axon‐Derived Netrin‐1 Instructs GABAergic Neuron Migration and Dopaminergic Substantia Nigra Development. Neuron, 107(684‐702), e689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

