Oxidative and glycolytic skeletal muscles deploy protective mechanisms to avoid atrophy under pathophysiological iron overload
- PMID: 35118832
- PMCID: PMC8978014
- DOI: 10.1002/jcsm.12897
Oxidative and glycolytic skeletal muscles deploy protective mechanisms to avoid atrophy under pathophysiological iron overload
Abstract
Background: Iron excess has been proposed as an essential factor in skeletal muscle wasting. Studies have reported correlations between muscle iron accumulation and atrophy, either through ageing or by using experimental models of secondary iron overload. However, iron treatments performed in most of these studies induced an extra-pathophysiological iron overload, more representative of intoxication or poisoning. The main objective of this study was to determine the impact of iron excess closer to pathophysiological conditions on structural and metabolic adaptations (i) in differentiated myotubes and (ii) in skeletal muscle exhibiting oxidative (i.e. the soleus) or glycolytic (i.e. the gastrocnemius) metabolic phenotypes.
Methods: The impact of iron excess was assessed in both in vitro and in vivo models. Murine differentiated myotubes were exposed to ferric ammonium citrate (FAC) (i.e. 10 and 50 μM) for the in vitro component. The in vivo model was achieved by a single iron dextran subcutaneous injection (1 g/kg) in mice. Four months after the injection, soleus and gastrocnemius muscles were harvested for analysis.
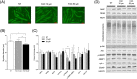
Results: In vitro, iron exposure caused dose-dependent increases of iron storage protein ferritin (P < 0.01) and dose-dependent decreases of mRNA TfR1 levels (P < 0.001), which support cellular adaptations to iron excess. Extra-physiological iron treatment (50 μM FAC) promoted myotube atrophy (P = 0.018), whereas myotube size remained unchanged under pathophysiological treatment (10 μM FAC). FAC treatments, whatever the doses tested, did not affect the expression of proteolytic markers (i.e. NF-κB, MurF1, and ubiquitinated proteins). In vivo, basal iron content and mRNA TfR1 levels were significantly higher in the soleus compared with the gastrocnemius (+130% and +127%; P < 0.001, respectively), supporting higher iron needs in oxidative skeletal muscle. Iron supplementation induced muscle iron accumulation in the soleus and gastrocnemius muscles (+79%, P < 0.001 and +34%, P = 0.002, respectively), but ferritin protein expression only increased in the gastrocnemius (+36%, P = 0.06). Despite iron accumulation, muscle weight, fibre diameter, and myosin heavy chain distribution remained unchanged in either skeletal muscle.
Conclusions: Together, these data support that under pathophysiological conditions, skeletal muscle can protect itself from the related deleterious effects of excess iron.
Keywords: Disuse; Mitochondria; Myosin heavy chain; Sarcopenia; Typology.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
No conflict of interest, financial or otherwise, is declared by the authors.
Figures
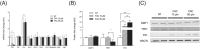
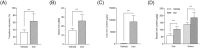



References
-
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003;51:451–458. - PubMed
-
- Pierre N, Appriou Z, Gratas‐Delamarche A, Derbré F. From physical inactivity to immobilization: dissecting the role of oxidative stress in skeletal muscle insulin resistance and atrophy. Free Radic Biol Med 2016;98:197–207. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

