Clinical Evaluation of a Novel Insulin Immunosensor
- PMID: 35118893
- PMCID: PMC10347985
- DOI: 10.1177/19322968221074406
Clinical Evaluation of a Novel Insulin Immunosensor
Abstract
Background: The estimation of available active insulin remains a limitation of automated insulin delivery systems. Currently, insulin pumps calculate active insulin using mathematical decay curves, while quantitative measurements of insulin would explicitly provide person-specific PK insulin dynamics to assess remaining active insulin more accurately, permitting more effective glucose control.
Methods: We performed the first clinical evaluation of an insulin immunosensor chip, providing near real-time measurements of insulin levels. In this study, we sought to determine the accuracy of the novel insulin sensor and assess its therapeutic risk and benefit by presenting a new tool developed to indicate the potential therapeutic consequences arising from inaccurate insulin measurements.
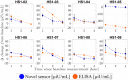
Results: Nine adult participants with type-1 diabetes completed the study. The change from baseline in immunosensor-measured insulin levels was compared with values obtained by standard enzyme-linked immunosorbant assay (ELISA) after preprandial injection of insulin. The point-of-care quantification of insulin levels revealed similar temporal trends as those from the laboratory insulin ELISA. The results showed that 70% of the paired immunosensor-reference values were concordant, which suggests that the patient could take action safely based on insulin concentration obtained by the novel sensor.
Conclusions: This proposed technology and preliminary feasibility evaluation show encouraging results for near real-time evaluation of insulin levels, with the potential to improve diabetes management. Real-time measurements of insulin provide person-specific insulin dynamics that could be used to make more informed decisions regarding insulin dosing, thus helping to prevent hypoglycemia and improve diabetes outcomes.
Keywords: immunosensor; insulin measurement; point-of-care; type-1 diabetes.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.E.P. is currently an employee and shareholder of Tandem Diabetes Care, Inc. The work presented in the manuscript was performed as part of his academic appointment at Sansum Diabetes Research Institute and is independent of his employment with Tandem Diabetes Care. E.D. reports receiving grants from JDRF, NIH, and Helmsley Charitable Trust, personal fees from Roche and Eli Lilly, patents on artificial pancreas technology, and product support from Dexcom, Insulet, Tandem, and Roche. E.D. is currently an employee and shareholder of Eli Lilly and Company. The work presented in this manuscript was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. F.J.D. reports equity, licensed IP and is a member of the Scientific Advisory Board of Mode AGC. L.M.L. reports grant support to her institution from NIH, JDRF, Helmsley Charitable Trust, Eli Lilly and Company, Insulet, Dexcom, and Boehringer Ingelheim; she receives consulting fees unrelated to the current report from Johnson & Johnson, Sanofi, NovoNordisk, Roche, Dexcom, Insulet, Boehringer Ingelheim, ConvaTec, Medtronic, Lifescan, Laxmi, and Insulogic. M.E.P. reports receiving grant support, provided to her institution, from NIH, Helmsley Charitable Trust, Chan Zuckerberg Foundation, and Dexcom, patents related to hypoglycemia and pump therapy for hypoglycemia, and advisory board fees from Fractyl (unrelated to the current report). F.T. and H.T. are currently employees of ActioX LLC. The work presented in the manuscript was performed as part of their academic appointment at UCSD. All other authors report no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

