The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer
- PMID: 35119068
- PMCID: PMC8819670
- DOI: 10.1042/CS20201474
The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer
Abstract
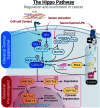
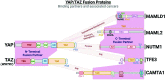
Tumorigenesis is a highly complex process, involving many interrelated and cross-acting signalling pathways. One such pathway that has garnered much attention in the field of cancer research over the last decade is the Hippo signalling pathway. Consisting of two antagonistic modules, the pathway plays an integral role in both tumour suppressive and oncogenic processes, generally via regulation of a diverse set of genes involved in a range of biological functions. This review discusses the history of the pathway within the context of cancer and explores some of the most recent discoveries as to how this critical transducer of cellular signalling can influence cancer progression. A special focus is on the various recent efforts to therapeutically target the key effectors of the pathway in both preclinical and clinical settings.
Keywords: AlphaFold; Cancer; Hippo pathway; Immuno-oncology; Mesothelioma; Sarcoma.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

