BACE1: More than just a β-secretase
- PMID: 35119166
- PMCID: PMC9286785
- DOI: 10.1111/obr.13430
BACE1: More than just a β-secretase
Abstract
β-site amyloid precursor protein cleaving enzyme-1 (BACE1) research has historically focused on its actions as the β-secretase responsible for the production of β-amyloid beta, observed in Alzheimer's disease. Although the greatest expression of BACE1 is found in the brain, BACE1 mRNA and protein is also found in many cell types including pancreatic β-cells, adipocytes, hepatocytes, and vascular cells. Pathologically elevated BACE1 expression in these cells has been implicated in the development of metabolic diseases, including type 2 diabetes, obesity, and cardiovascular disease. In this review, we examine key questions surrounding the BACE1 literature, including how is BACE1 regulated and how dysregulation may occur in disease, and understand how BACE1 regulates metabolism via cleavage of a myriad of substrates. The phenotype of the BACE1 knockout mice models, including reduced weight gain, increased energy expenditure, and enhanced leptin signaling, proposes a physiological role of BACE1 in regulating energy metabolism and homeostasis. Taken together with the weight loss observed with BACE1 inhibitors in clinical trials, these data highlight a novel role for BACE1 in regulation of metabolic physiology. Finally, this review aims to examine the possibility that BACE1 inhibitors could provide a innovative treatment for obesity and its comorbidities.
Keywords: BACE1; obesity; type 2 diabetes.
© 2022 The Authors. Obesity Reviews published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
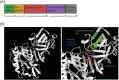



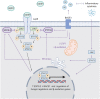
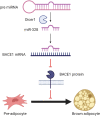
References
-
- Yan R, Bienkowski MJ, Shuck ME, et al. Membrane‐anchored aspartyl protease with Alzheimer's disease β‐secretase activity. Nature. 1999;402(6761):533‐537. - PubMed
-
- Vassar R. Beta‐secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735‐741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

