Novel insights from a multiomics dissection of the Hayflick limit
- PMID: 35119359
- PMCID: PMC8933007
- DOI: 10.7554/eLife.70283
Novel insights from a multiomics dissection of the Hayflick limit
Abstract
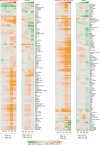
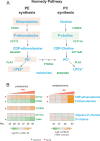
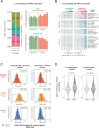
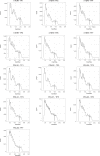
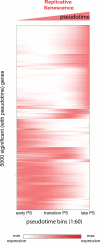
The process wherein dividing cells exhaust proliferative capacity and enter into replicative senescence has become a prominent model for cellular aging in vitro. Despite decades of study, this cellular state is not fully understood in culture and even much less so during aging. Here, we revisit Leonard Hayflick's original observation of replicative senescence in WI-38 human lung fibroblasts equipped with a battery of modern techniques including RNA-seq, single-cell RNA-seq, proteomics, metabolomics, and ATAC-seq. We find evidence that the transition to a senescent state manifests early, increases gradually, and corresponds to a concomitant global increase in DNA accessibility in nucleolar and lamin associated domains. Furthermore, we demonstrate that senescent WI-38 cells acquire a striking resemblance to myofibroblasts in a process similar to the epithelial to mesenchymal transition (EMT) that is regulated by t YAP1/TEAD1 and TGF-β2. Lastly, we show that verteporfin inhibition of YAP1/TEAD1 activity in aged WI-38 cells robustly attenuates this gene expression program.
Keywords: chromosomes; epithelial to mesenchymal transition; gene expression; genetics; genomics; human; myofibroblast; replicative senescence.
© 2022, Chan et al.
Conflict of interest statement
MC, HY, IS, TM, RW, AI, JO, JG, LC, TV, AF, CK, BB, FM, DK, MR, RC, AL, DB, DH is affiliated with Calico Life Sciences, LLC. The author has no other competing interests to declare
Figures











Comment in
-
A gradual path to mortality.Elife. 2022 Mar 18;11:e77749. doi: 10.7554/eLife.77749. Elife. 2022. PMID: 35302485 Free PMC article.
References
-
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology. 2013;15:978–990. doi: 10.1038/ncb2784. - DOI - PMC - PubMed
-
- Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. - DOI - PMC - PubMed
-
- Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, Kulick A, Houlihan S, Peerschke E, Friedman SL, Ponomarev V, Piersigilli A, Sadelain M, Lowe SW. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. - DOI - PMC - PubMed
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

