SNARE proteins: zip codes in vesicle targeting?
- PMID: 35119456
- PMCID: PMC8883487
- DOI: 10.1042/BCJ20210719
SNARE proteins: zip codes in vesicle targeting?
Abstract
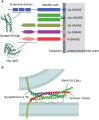
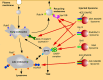
Membrane traffic in eukaryotic cells is mediated by transport vesicles that bud from a precursor compartment and are transported to their destination compartment where they dock and fuse. To reach their intracellular destination, transport vesicles contain targeting signals such as Rab GTPases and polyphosphoinositides that are recognized by tethering factors in the cytoplasm and that connect the vesicles with their respective destination compartment. The final step, membrane fusion, is mediated by SNARE proteins. SNAREs are connected to targeting signals and tethering factors by multiple interactions. However, it is still debated whether SNAREs only function downstream of targeting and tethering or whether they also participate in regulating targeting specificity. Here, we review the evidence and discuss recent data supporting a role of SNARE proteins as targeting signals in vesicle traffic.
Keywords: membrane fusion; snare proteins; trafficking.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

