Randomized Phase 3 LEAP-012 Study: Transarterial Chemoembolization With or Without Lenvatinib Plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma Not Amenable to Curative Treatment
- PMID: 35119481
- PMCID: PMC8940827
- DOI: 10.1007/s00270-021-03031-9
Randomized Phase 3 LEAP-012 Study: Transarterial Chemoembolization With or Without Lenvatinib Plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma Not Amenable to Curative Treatment
Abstract
Purpose: Transarterial chemoembolization (TACE) is the standard of care for patients with intermediate-stage hepatocellular carcinoma (HCC). Lenvatinib, a multikinase inhibitor, and pembrolizumab, a PD-1 inhibitor, have shown efficacy and tolerability in patients with HCC, and adding this combination to TACE may enhance clinical benefit.
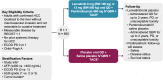
Protocol: LEAP-012 is a prospective, double-blind randomized phase 3 study. Adults with confirmed HCC localized to the liver without portal vein thrombosis and not amenable to curative treatment, ≥ 1 measurable tumor per Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1), Eastern Cooperative Oncology Group performance status 0 or 1, Child-Pugh class A and no previous systemic treatment for HCC are eligible. Patients will be randomly assigned to lenvatinib once daily plus pembrolizumab every 6 weeks plus TACE or placebos plus TACE. Dual primary endpoints are overall survival and progression-free survival per RECIST 1.1 by blinded independent central review (BICR). Secondary endpoints are progression-free survival, objective response rate, disease control rate, duration of response and time to progression per modified RECIST by BICR; objective response rate, disease control rate, duration of response and time to progression per RECIST 1.1 by BICR; and safety.
Statistics: The planned sample size, 950 patients, was calculated to permit accumulation of sufficient overall survival events in 5 years to achieve 90% power for the overall survival primary endpoint.
Discussion: LEAP-012 will evaluate the clinical benefit of adding lenvatinib plus pembrolizumab to TACE in patients with intermediate-stage HCC not amenable to curative treatment.
Clinicaltrials: gov NCT04246177.
Keywords: Intermediate-stage hepatocellular carcinoma; Lenvatinib; Pembrolizumab; Transarterial chemoembolization.
© 2022. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, N.J., U.S.A., Josep M. Llovet, Arndt Vogel, David C. Madoff, Richard S. Finn, Sadahisa Ogasawara, Zhenggang Ren, Kalgi Mody, Masatoshi Kudo.
Figures
References
-
- Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2017.1747. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

