Comparison of characteristics and tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18
- PMID: 35119498
- PMCID: PMC9374793
- DOI: 10.1007/s00262-022-03161-0
Comparison of characteristics and tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18
Abstract
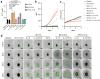
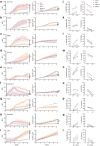
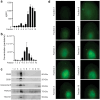
NK cell-based therapies have shown promise for hematological cancer forms, but their use against solid tumors is hampered by their poor ability to infiltrate the tumor. NK cells release extracellular vesicles (EVs) containing cytolytic proteins, indicating that NK-cell derived EVs may have therapeutic potential. In this study, we compared the tumor-targeting potential of EVs derived from either primary NK cells or the NK cell lines NK-92 and KHYG-1 cultured in IL-15 alone or in combination with IL-12 and IL-18. Primary NK cells were also stimulated through the activating receptor CD16. Tumor cell apoptosis was measured using a panel of human colon, melanoma, glioblastoma, prostate, breast, and ovarian tumor cell line spheroids. NK cells or NK-92 cells stimulated with IL-12, IL-15, and IL-18 generated EVs with higher efficiency than EVs from resting cells, although similar amounts of EVs were produced under both conditions. Proteomic analysis indicated similar distribution of cytolytic proteins in EVs from primary NK cells and NK-92, but lower levels in KHYG-1 EVs that translated into poor capacity for KHYG-1 EVs at targeting tumor cell lines. Further, we show that CD16-stimulated NK cells release low amounts of EVs devoid of cytolytic proteins. Importantly, EVs from cytokine-stimulated NK cells penetrate into the spheroid core, and tumor spheroid susceptibility to NK-cell derived EVs was linked to differential expression of the NKG2D ligands MICA/B, which was blocked with an anti-NKG2D antibody. We conclude that EVs from activated primary NK cells or NK-92 cells has the best potential to infiltrate and target solid tumors.
Keywords: EVs; IL-12; IL-15; IL-18; NK cells; NKG2D.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

