"De-Shrinking" EBEs: The Solution for Bayesian Therapeutic Drug Monitoring
- PMID: 35119624
- PMCID: PMC9095561
- DOI: 10.1007/s40262-021-01105-y
"De-Shrinking" EBEs: The Solution for Bayesian Therapeutic Drug Monitoring
Abstract
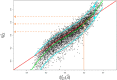
Background: Therapeutic drug monitoring (TDM) aims at individualising a dosage regimen and is increasingly being performed by estimating individual pharmacokinetic parameters via empirical Bayes estimates (EBEs). However, EBEs suffer from shrinkage that makes them biased. This bias is a weakness for TDM and probably a barrier to the acceptance of drug dosage adjustments by prescribers.
Objective: The aim of this article is to propose a methodology that allows a correction of EBE shrinkage and an improvement in their precision.
Methods: As EBEs are defined, they can be seen as a special case of ridge estimators depending on a parameter usually denoted λ. After a bias correction depending on λ, we chose λ so that the individual pharmacokinetic estimations have minimal imprecision. Our estimate is by construction always better than EBE with respect to bias (i.e. shrinkage) and precision.
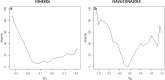
Results: We illustrate the performance of this approach with two different drugs: iohexol and isavuconazole. Depending on the patient's actual pharmacokinetic parameter values, the improvement given by our approach ranged from 0 to 100%.
Conclusion: This innovative methodology is promising since, to the best of our knowledge, no other individual shrinkage correction has been proposed.
© 2022. The Author(s).
Conflict of interest statement
Sarah Baklouti, Peggy Gandia, and Didier Concordet have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Figures

References
-
- Schumacher GE, Barr JT. Bayesian approaches in pharmacokinetic decision making. Clin Pharm. 1984;3:525–530. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

