Patterns of eukaryotic diversity from the surface to the deep-ocean sediment
- PMID: 35119936
- PMCID: PMC8816347
- DOI: 10.1126/sciadv.abj9309
Patterns of eukaryotic diversity from the surface to the deep-ocean sediment
Abstract
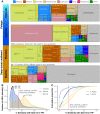
Remote deep-ocean sediment (DOS) ecosystems are among the least explored biomes on Earth. Genomic assessments of their biodiversity have failed to separate indigenous benthic organisms from sinking plankton. Here, we compare global-scale eukaryotic DNA metabarcoding datasets (18S-V9) from abyssal and lower bathyal surficial sediments and euphotic and aphotic ocean pelagic layers to distinguish plankton from benthic diversity in sediment material. Based on 1685 samples collected throughout the world ocean, we show that DOS diversity is at least threefold that in pelagic realms, with nearly two-thirds represented by abundant yet unknown eukaryotes. These benthic communities are spatially structured by ocean basins and particulate organic carbon (POC) flux from the upper ocean. Plankton DNA reaching the DOS originates from abundant species, with maximal deposition at high latitudes. Its seafloor DNA signature predicts variations in POC export from the surface and reveals previously overlooked taxa that may drive the biological carbon pump.
Figures




References
-
- Thurber A. R., Sweetman A. K., Narayanaswamy B. E., Jones D. O. B., Ingels J., Hansman R. L., Ecosystem function and services provided by the deep sea. Biogeosciences 11, 3941–3963 (2014).
-
- Levin L. A., Le Bris N., The deep ocean under climate change. Science 350, 766–768 (2015). - PubMed
-
- Breitburg D., Levin L. A., Oschlies A., Grégoire M., Chavez F. P., Conley D. J., Garçon V., Gilbert D., Gutiérrez D., Isensee K., Jacinto G. S., Limburg K. E., Montes I., Naqvi S. W. A., Pitcher G. C., Rabalais N. N., Roman M. R., Rose K. A., Seibel B. A., Telszewski M., Yasuhara M., Zhang J., Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018). - PubMed
-
- Smith C. R., Tunnicliffe V., Colaço A., Drazen J. C., Gollner S., Levin L. A., Mestre N. C., Metaxas A., Molodtsova T. N., Morato T., Sweetman A. K., Washburn T., Amon D. J., Deep-sea misconceptions cause underestimation of seabed-mining impacts. Trends Ecol. Evol. 35, 853–857 (2020). - PubMed
LinkOut - more resources
Full Text Sources

