Lung and blood early biomarkers for host-directed tuberculosis therapies: Secondary outcome measures from a randomized controlled trial
- PMID: 35120127
- PMCID: PMC8815935
- DOI: 10.1371/journal.pone.0252097
Lung and blood early biomarkers for host-directed tuberculosis therapies: Secondary outcome measures from a randomized controlled trial
Abstract
Background: Current tuberculosis treatments leave most patients with bronchiectasis and fibrosis, permanent conditions that impair lung function and increase all-cause post-TB mortality. Host-directed therapies (HDTs) may reduce lung inflammation and hasten eradication of infection. Biomarkers can accelerate tuberculosis regimen development, but no studies have yet examined early biomarkers of TB-HDTs.
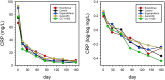
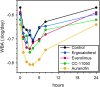
Methods: Biomarkers of inflammation and microbicidal activity were evaluated as a part of a recent phase-2 randomized controlled trial of four HDTs in 200 patients with pulmonary tuberculosis and baseline predictors of poor outcome, including CC-11050 (PDE4i), everolimus (mTORi), auranofin (oral gold salt), and ergocalciferol (vitamin D). Two of the 4 arms (CC-11050 and everolimus) showed superior recovery of lung function at day 180 compared to control; none showed accelerated eradication of MTB infection. Patients underwent 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) on entry and day 56. PET signals were analyzed according to total, maximal, and peak glycolytic activity; CT was analyzed according to total modified Hounsfield units to assess radiodensity. Mycobactericidal activity in ex vivo whole blood culture was measured on days 42, 84, and 140. C-reactive protein (CRP) was measured at multiple time points.
Results: All PET/CT parameters showed highly significant reductions from baseline to day 56; however, only maximal or peak glycolytic activity showed further experimental reduction compared to controls, and only in everolimus recipients. CRP dropped precipitously during early treatment, but did so equally in all arms; over the entire period of treatment, the rate of decline of CRP tended to be greater in CC-11050 recipients than in controls but this fell short of statistical significance. Whole blood mycobactericidal activity in ex-vivo culture was enhanced by auranofin compared to controls, but not by other HDTs.
Conclusions: None of these early biomarkers correctly predicted HDT effects on inflammation or infection across all four experimental arms. Instead, they each appear to show highly specific responses related to HDT mechanisms of action.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Global tuberculosis report. Geneva: 2019 WHO/CDS/TB/2019.15.
-
- Duong M, Islam S, Rangarajan S, Leong D, Kurmi O, Teo K, et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health. 2019;7(5):e613–e23. doi: 10.1016/S2214-109X(19)30070-1 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

