Beyond rates: time-varying dynamics of high frequency oscillations as a biomarker of the seizure onset zone
- PMID: 35120337
- PMCID: PMC9258635
- DOI: 10.1088/1741-2552/ac520f
Beyond rates: time-varying dynamics of high frequency oscillations as a biomarker of the seizure onset zone
Abstract
Objective. High frequency oscillations (HFOs) recorded by intracranial electrodes have generated excitement for their potential to help localize epileptic tissue for surgical resection. However, the number of HFOs per minute (i.e. the HFO 'rate') is not stable over the duration of intracranial recordings; for example, the rate of HFOs increases during periods of slow-wave sleep. Moreover, HFOs that are predictive of epileptic tissue may occur in oscillatory patterns due to phase coupling with lower frequencies. Therefore, we sought to further characterize between-seizure (i.e. 'interictal') HFO dynamics both within and outside the seizure onset zone (SOZ).Approach. Using long-term intracranial EEG (mean duration 10.3 h) from 16 patients, we automatically detected HFOs using a new algorithm. We then fit a hierarchical negative binomial model to the HFO counts. To account for differences in HFO dynamics and rates between sleep and wakefulness, we also fit a mixture model to the same data that included the ability to switch between two discrete brain states that were automatically determined during the fitting process. The ability to predict the SOZ by model parameters describing HFO dynamics (i.e. clumping coefficients and coefficients of variation) was assessed using receiver operating characteristic curves.Main results. Parameters that described HFO dynamics were predictive of SOZ. In fact, these parameters were found to be more consistently predictive than HFO rate. Using concurrent scalp EEG in two patients, we show that the model-found brain states corresponded to (1) non-REM sleep and (2) awake and rapid eye movement sleep. However the brain state most likely corresponding to slow-wave sleep in the second model improved SOZ prediction compared to the first model for only some patients.Significance. This work suggests that delineation of SOZ with interictal data can be improved by the inclusion of time-varying HFO dynamics.
Keywords: epilepsy; epileptogenic zone; hierarchical Bayesian methods; high-frequency oscillations (HFOs); intracranial EEG; ripple; surgery.
Creative Commons Attribution license.
Conflict of interest statement
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
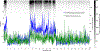


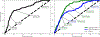



References
-
- Bendat JS and Piersol AG (2011). Random data: analysis and measurement procedures, volume 729. John Wiley & Sons.
-
- Bernardo D, Nariai H, Hussain SA, Sankar R, Salamon N, Krueger DA, Sahin M, Northrup H, Bebin EM, Wu JY, et al. (2018). Visual and semi-automatic non-invasive detection of interictal fast ripples: A potential biomarker of epilepsy in children with tuberous sclerosis complex. Clinical Neurophysiology, 129(7):1458–1466. - PubMed
-
- Blanco JA, Stead M, Krieger A, Viventi J, Marsh WR, Lee KH, Worrell GA, and Litt B. (2010). Unsupervised Classification of High-Frequency Oscillations in Human Neocortical Epilepsy and Control Patients. Journal of Neurophysiology, 104(5):2900–2912. Publisher: American Physiological Society. - PMC - PubMed
