Parsing the Network Mechanisms of Electroconvulsive Therapy
- PMID: 35120710
- PMCID: PMC9196257
- DOI: 10.1016/j.biopsych.2021.11.016
Parsing the Network Mechanisms of Electroconvulsive Therapy
Abstract
Electroconvulsive therapy (ECT) is one of the oldest and most effective forms of neurostimulation, wherein electrical current is used to elicit brief, generalized seizures under general anesthesia. When electrodes are positioned to target frontotemporal cortex, ECT is arguably the most effective treatment for severe major depression, with response rates and times superior to other available antidepressant therapies. Neuroimaging research has been pivotal in improving the field's mechanistic understanding of ECT, with a growing number of magnetic resonance imaging studies demonstrating hippocampal plasticity after ECT, in line with evidence of upregulated neurotrophic processes in the hippocampus in animal models. However, the precise roles of the hippocampus and other brain regions in antidepressant response to ECT remain unclear. Seizure physiology may also play a role in antidepressant response to ECT, as indicated by early positron emission tomography, single-photon emission computed tomography, and electroencephalography research and corroborated by recent magnetic resonance imaging studies. In this review, we discuss the evidence supporting neuroplasticity in the hippocampus and other brain regions during and after ECT, and their associations with antidepressant response. We also offer a mechanistic, circuit-level model that proposes that core mechanisms of antidepressant response to ECT involve thalamocortical and cerebellar networks that are active during seizure generalization and termination over repeated ECT sessions, and their interactions with corticolimbic circuits that are dysfunctional prior to treatment and targeted with the electrical stimulus.
Keywords: Antidepressant; Depression; Electroconvulsive therapy; MRI; Neuroimaging; Seizure.
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
All authors report no biomedical financial interests or potential conflicts of interest.
Figures


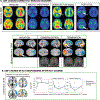
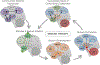
References
-
- Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK (2012): Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14: 146–150. - PubMed
-
- Fink M (2014): What was learned: studies by the consortium for research in ECT (CORE) 1997–2011. Acta Psychiatr Scand 129: 417–426. - PubMed
-
- Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. (2004): Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 65: 485–491. - PubMed
-
- Kellner CH, Pritchett JT, Beale MD, Coffey CE (1997): Handbook of ECT. Washington, D.C.: American Psychiatric Press.
-
- Lisanby SH, Maddox JH, Prudic J, Devanand DP, Sackeim HA (2000): The Effects of Electroconvulsive Therapy on Memory of Autobiographical and Public Events. Arch Gen Psychiatry 57: 581–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

