Reverse-Transcription Loop-Mediated Isothermal Amplification Has High Accuracy for Detecting Severe Acute Respiratory Syndrome Coronavirus 2 in Saliva and Nasopharyngeal/Oropharyngeal Swabs from Asymptomatic and Symptomatic Individuals
- PMID: 35121140
- PMCID: PMC8806713
- DOI: 10.1016/j.jmoldx.2021.12.007
Reverse-Transcription Loop-Mediated Isothermal Amplification Has High Accuracy for Detecting Severe Acute Respiratory Syndrome Coronavirus 2 in Saliva and Nasopharyngeal/Oropharyngeal Swabs from Asymptomatic and Symptomatic Individuals
Abstract
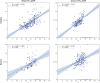
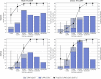
Previous studies have described reverse-transcription loop-mediated isothermal amplification (RT-LAMP) for the rapid detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal/oropharyngeal swab and saliva samples. This multisite clinical evaluation describes the validation of an improved sample preparation method for extraction-free RT-LAMP and reports clinical performance of four RT-LAMP assay formats for SARS-CoV-2 detection. Direct RT-LAMP was performed on 559 swabs and 86,760 saliva samples and RNA RT-LAMP on extracted RNA from 12,619 swabs and 12,521 saliva samples from asymptomatic and symptomatic individuals across health care and community settings. For direct RT-LAMP, overall diagnostic sensitivity (DSe) was 70.35% (95% CI, 63.48%-76.60%) on swabs and 84.62% (95% CI, 79.50%-88.88%) on saliva, with diagnostic specificity of 100% (95% CI, 98.98%-100.00%) on swabs and 100% (95% CI, 99.72%-100.00%) on saliva, compared with quantitative RT-PCR (RT-qPCR); analyzing samples with RT-qPCR ORF1ab CT values of ≤25 and ≤33, DSe values were 100% (95% CI, 96.34%-100%) and 77.78% (95% CI, 70.99%-83.62%) for swabs, and 99.01% (95% CI, 94.61%-99.97%) and 87.61% (95% CI, 82.69%-91.54%) for saliva, respectively. For RNA RT-LAMP, overall DSe and diagnostic specificity were 96.06% (95% CI, 92.88%-98.12%) and 99.99% (95% CI, 99.95%-100%) for swabs, and 80.65% (95% CI, 73.54%-86.54%) and 99.99% (95% CI, 99.95%-100%) for saliva, respectively. These findings demonstrate that RT-LAMP is applicable to a variety of use cases, including frequent, interval-based direct RT-LAMP of saliva from asymptomatic individuals who may otherwise be missed using symptomatic testing alone.
Copyright © 2022 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Colorimetric RT-LAMP for SARS-CoV-2 detection from nasopharyngeal swabs or crude saliva: a multicountry diagnostic accuracy study in Africa.Lancet Glob Health. 2025 Jul;13(7):e1258-e1267. doi: 10.1016/S2214-109X(25)00150-0. Lancet Glob Health. 2025. PMID: 40580991 Free PMC article.
-
Diagnostic accuracy of loop-mediated isothermal amplification coupled to nanopore sequencing (LamPORE) for the detection of SARS-CoV-2 infection at scale in symptomatic and asymptomatic populations.Clin Microbiol Infect. 2021 Sep;27(9):1348.e1-1348.e7. doi: 10.1016/j.cmi.2021.04.008. Epub 2021 Apr 24. Clin Microbiol Infect. 2021. PMID: 33901668 Free PMC article.
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
Evaluation of RT-LAMP Assay for Rapid Detection of SARS-CoV-2.Lab Med. 2023 Jan 5;54(1):56-64. doi: 10.1093/labmed/lmac030. Lab Med. 2023. PMID: 35849098 Free PMC article.
Cited by
-
Validation of a rapid, saliva-based, and ultra-sensitive SARS-CoV-2 screening system for pandemic-scale infection surveillance.Sci Rep. 2022 Apr 8;12(1):5936. doi: 10.1038/s41598-022-08263-4. Sci Rep. 2022. PMID: 35395856 Free PMC article.
-
Rapid SARS-CoV-2 Detection Using the Lucira™ Check It COVID-19 Test Kit.Diagnostics (Basel). 2022 Aug 3;12(8):1877. doi: 10.3390/diagnostics12081877. Diagnostics (Basel). 2022. PMID: 36010227 Free PMC article.
-
RUNCOV: a one-pot triplex real-time RT-LAMP as a point-of-care diagnostic tool for detecting SARS-CoV-2.Biol Methods Protoc. 2025 Feb 7;10(1):bpaf010. doi: 10.1093/biomethods/bpaf010. eCollection 2025. Biol Methods Protoc. 2025. PMID: 40046730 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
[Diagnosis by isothermal amplification of nucleic acids. Opportunity for community pharmacies].Farm Comunitarios. 2024 Apr 11;16(2):46-53. doi: 10.33620/FC.2173-9218.(2024).14. eCollection 2024 Apr 15. Farm Comunitarios. 2024. PMID: 39156031 Free PMC article. Review. Spanish.
References
-
- Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y., Akdis C., Gao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. - PubMed
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., Van Der Veer B., Van Den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:23–30. - PMC - PubMed
-
- Fowler V.L., Armson B., Gonzales J.L., Wise E.L., Howson E.L.A., Vincent-Mistiaen Z., Fouch S., Maltby C.J., Grippon S., Munro S., Jones L., Holmes T., Tillyer C., Elwell J., Sowood A., de Peyer O., Dixon S., Hatcher T., Patrick H., Laxman S., Walsh C., Andreou M., Morant N., Clark D., Moore N., Houghton R., Cortes N.J., Kidd S.P. A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection. J Infect. 2020;82:117–125. - PMC - PubMed
-
- de Paula Eduardo F., Bezinelli L.M., de Araujo C.A.R., Moraes J.V.V., Birbrair A., Pinho J.R.R., Hamerschlak N., Al-Hashimi I., Heller D. Self-collected unstimulated saliva, oral swab, and nasopharyngeal swab specimens in the detection of SARS-CoV-2. Clin Oral Investig. 2022;26:1561–1567. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- MC_UP_A620_1015/MRC_/Medical Research Council/United Kingdom
- MC_PC_21003/MRC_/Medical Research Council/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MC_PC_21001/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/4/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- MC_PC_21000/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

