Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study
- PMID: 35121639
- PMCID: PMC8819784
- DOI: 10.1136/rmdopen-2021-002012
Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study
Erratum in
-
Correction: Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study.RMD Open. 2023 Jun;9(2):e002012corr1. doi: 10.1136/rmdopen-2021-002012corr1. RMD Open. 2023. PMID: 37321671 Free PMC article. No abstract available.
Abstract
Objectives: To assess the long-term safety and efficacy of the Janus kinase inhibitor upadacitinib versus adalimumab over 3 years in the ongoing long-term extension (LTE) of SELECT-COMPARE, a randomised controlled phase 3 trial of patients with active rheumatoid arthritis and inadequate response to methotrexate (MTX).
Methods: Patients on stable background MTX were randomised 2:2:1 to upadacitinib 15 mg, placebo or adalimumab 40 mg. Patients with an insufficient response were switched by week 26 from placebo to upadacitinib, upadacitinib to adalimumab or adalimumab to upadacitinib. Patients who completed the 48-week double-blind period could enter an LTE for up to 10 years. Safety and efficacy results were analysed here through 3 years. Treatment-emergent adverse events (AEs) were summarised based on exposure to upadacitinib and adalimumab. Efficacy was analysed by original randomised groups (non-responder imputation), as well as separately by treatment sequence (as observed).
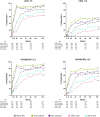
Results: Rates of several AEs were generally comparable between upadacitinib and adalimumab, including AEs leading to discontinuation, serious infections and serious AEs, malignancies, major adverse cardiac events, venous thromboembolism and deaths. Consistent with earlier results, herpes zoster, lymphopaenia, hepatic disorder and CPK elevation were reported at higher rates with upadacitinib versus adalimumab. In terms of efficacy, upadacitinib continued to show numerically better clinical responses than adalimumab over 3 years across all endpoints, including low disease activity and remission.
Conclusion: The safety profile of UPA 15 mg was consistent with previous study-specific and integrated safety reports. Higher levels of clinical response continued to be observed with upadacitinib versus adalimumab through 3 years of treatment.
Keywords: adalimumab; arthritis; cardiovascular diseases; rheumatoid; therapeutics; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RF: Research grants and consulting fees from AbbVie, Amgen, Astra-Zeneca, Biosplice, BMS, Flexion, Galvani, Genentech, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi-Aventis, Teva, UCB, Viela, Vorso. EM: Research grants and consulting fees from AbbVie, Lilly, Pfizer, Roche, BMS, Sandoz, Amgen, AstraZeneca, GSK, Janssen, Novartis. LB: speaker, consulting fees and research support from Amgen, BMS, Janssen, Roche, UCB, AbbVie, Pfizer, Merck, Sanofi, Eli Lilly, Novartis, Teva, Gilead, Fresenius Kabi. CGP: Employee and shareholder: Spire Sciences; speaker: Amgen, Bristol-Myers Squibb; consultant: Aclaris, Centrexion, Daiichi-Sankyo, EMD Serono, Five Prime, Flexion Therapeutics, Genentech, Gilead, GlaxoSmithKline, Istresso, Eli Lilly, Myriad Genetics, Novartis, Roche, SetPoint, Sorrento, UCB. PD: Speaker fees from BMS, Sanofi, Eli Lilly, Celltrion. YT: speaker and/or received honoraria from Gilead, AbbVie, Boehringer Ingelheim, Eli Lilly, Mitsubishi-Tanabe, Chugai, Amgen, YL Biologics, Eisai, Astellas, Bristol-Myers, AstraZeneca; research grants from Asahi-Kasei, AbbVie, Chugai, Mitsubishi Tanabe, Eisai, Takeda, Corrona, Daiichi-Sankyo, Kowa, Boehringer Ingelheim. JS: Speaker, research grants and consulting fees from AbbVie, Sandoz, Pfizer, Roche, BMS, UCB, MSD, Accord, Janssen. NK, XB, YL, and I-HS: employees of AbbVie and may hold stock or options.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
