Evaluating the efficacy of a priming dose of cyclophosphamide prior to pembrolizumab to treat metastatic triple negative breast cancer
- PMID: 35121644
- PMCID: PMC8819787
- DOI: 10.1136/jitc-2021-003427
Evaluating the efficacy of a priming dose of cyclophosphamide prior to pembrolizumab to treat metastatic triple negative breast cancer
Abstract
Purpose: Triple negative breast cancer (TNBC) is characterized by the presence of immune cells in the tumor microenvironment, however, the response to single-agent immune checkpoint inhibitor (ICI) therapy is modest. Preclinical models have demonstrated that intratumoral regulatory T cells (Tregs) dampen the antitumor response to ICI. We performed a single-arm phase II trial to evaluate the efficacy of a single low dose of cyclophosphamide (Cy) to deplete Tregs administered before initiating pembrolizumab.
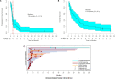
Patients and methods: 40 patients with pretreated metastatic TNBC were enrolled. The primary endpoints were progression-free survival (PFS) and change in peripheral blood Tregs after Cy. Secondary endpoints included overall response rate (ORR), duration of response, overall survival, treatment-related adverse events (AEs), and correlative evaluations.
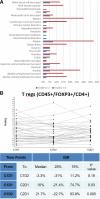
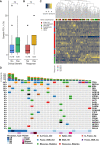
Results: Median PFS was 1.8 months, and the ORR was 21%. Tregs were not significantly decreased after Cy prior to ICI (-3.3%, p=0.19), and increased significantly after the first cycle of therapy (+21% between cycles 1 and 2, p=0.005). Immune-related AEs were similar to historical pembrolizumab monotherapy, and were associated with response to therapy (p=0.02). Patients with pretreatment tumors harboring increased expression of B cell metagene signatures and increased circulating B cell receptor repertoire diversity were associated with clinical response and immune-related toxicity (IRT).
Conclusions: Among patients with heavily pretreated TNBC, Cy prior to pembrolizumab did not significantly deplete Tregs, and in those with decreased numbers there was rapid recovery following therapy. Increased B cell gene expression in baseline samples was associated with clinical response and IRT.
Keywords: breast neoplasms; clinical trials; immunotherapy; phase II as topic; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CKA receives research funding from PUMA, Lilly, MSD, Seattle Genetics, Nektar, Tesaro, and G1 Therapeutics, ZION, Novartis, Pfizer; compensation for consulting from Genentech, Eisai, IPSEN, Seattle Genetics, AstraZeneca, Novartis; and royalties from UpToDate and Jones and Bartlett. BV holds equity in GeneCentric Therapeutics. CP is an equity stockholder and consultant of BioClassifier, and an equity stock holder, consultant, and Board of Directors member of GeneCentric Therapeutics. CP is also listed as an inventor on patent applications for the Breast PAM50 assay. JS receives funding from MSD, GSK, and Carisma, is a scientific consultant for PIQUE Therapeutics and has filed IP for the use of STING agonists to enhance CAR T cell for breast cancer. The other authors have no conflicts requiring disclosure.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
