A dominant negative variant of RAB5B disrupts maturation of surfactant protein B and surfactant protein C
- PMID: 35121658
- PMCID: PMC8832968
- DOI: 10.1073/pnas.2105228119
A dominant negative variant of RAB5B disrupts maturation of surfactant protein B and surfactant protein C
Abstract
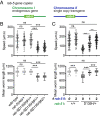
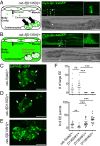
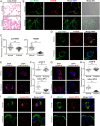
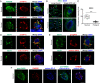
Pathogenic variants in surfactant proteins SP-B and SP-C cause surfactant deficiency and interstitial lung disease. Surfactant proteins are synthesized as precursors (proSP-B, proSP-C), trafficked, and processed via a vesicular-regulated secretion pathway; however, control of vesicular trafficking events is not fully understood. Through the Undiagnosed Diseases Network, we evaluated a child with interstitial lung disease suggestive of surfactant deficiency. Variants in known surfactant dysfunction disorder genes were not found in trio exome sequencing. Instead, a de novo heterozygous variant in RAB5B was identified in the Ras/Rab GTPases family nucleotide binding domain, p.Asp136His. Functional studies were performed in Caenorhabditis elegans by knocking the proband variant into the conserved position (Asp135) of the ortholog, rab-5 Genetic analysis demonstrated that rab-5[Asp135His] is damaging, producing a strong dominant negative gene product. rab-5[Asp135His] heterozygotes were also defective in endocytosis and early endosome (EE) fusion. Immunostaining studies of the proband's lung biopsy revealed that RAB5B and EE marker EEA1 were significantly reduced in alveolar type II cells and that mature SP-B and SP-C were significantly reduced, while proSP-B and proSP-C were normal. Furthermore, staining normal lung showed colocalization of RAB5B and EEA1 with proSP-B and proSP-C. These findings indicate that dominant negative-acting RAB5B Asp136His and EE dysfunction cause a defect in processing/trafficking to produce mature SP-B and SP-C, resulting in interstitial lung disease, and that RAB5B and EEs normally function in the surfactant secretion pathway. Together, the data suggest a noncanonical function for RAB5B and identify RAB5B p.Asp136His as a genetic mechanism for a surfactant dysfunction disorder.
Keywords: Caenorhabditis elegans; RAB5B; endocytosis; surfactant dysfunction disorder; surfactant proteins.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics Laboratories.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

