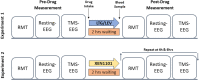
Spontaneous and TMS-related EEG changes as new biomarkers to measure anti-epileptic drug effects
- PMID: 35121751
- PMCID: PMC8817040
- DOI: 10.1038/s41598-022-05179-x
Spontaneous and TMS-related EEG changes as new biomarkers to measure anti-epileptic drug effects
Abstract
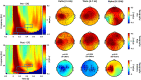
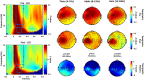
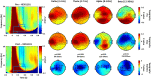
Robust biomarkers for anti-epileptic drugs (AEDs) activity in the human brain are essential to increase the probability of successful drug development. The frequency analysis of electroencephalographic (EEG) activity, either spontaneous or evoked by transcranial magnetic stimulation (TMS-EEG) can provide cortical readouts for AEDs. However, a systematic evaluation of the effect of AEDs on spontaneous oscillations and TMS-related spectral perturbation (TRSP) has not yet been provided. We studied the effects of Lamotrigine, Levetiracetam, and of a novel potassium channel opener (XEN1101) in two groups of healthy volunteers. Levetiracetam suppressed TRSP theta, alpha and beta power, whereas Lamotrigine decreased delta and theta but increased the alpha power. Finally, XEN1101 decreased TRSP delta, theta, alpha and beta power. Resting-state EEG showed a decrease of theta band power after Lamotrigine intake. Levetiracetam increased theta, beta and gamma power, while XEN1101 produced an increase of delta, theta, beta and gamma power. Spontaneous and TMS-related cortical oscillations represent a powerful tool to characterize the effect of AEDs on in vivo brain activity. Spectral fingerprints of specific AEDs should be further investigated to provide robust and objective biomarkers of biological effect in human clinical trials.
© 2022. The Author(s).
Conflict of interest statement
The author GNB declares a conflict of interest, as an employee of Xenon Pharmaceuticals. Inc. Burnaby Canada (
Figures