Ferredoxin reductase and p53 are necessary for lipid homeostasis and tumor suppression through the ABCA1-SREBP pathway
- PMID: 35121827
- PMCID: PMC8933276
- DOI: 10.1038/s41388-021-02100-0
Ferredoxin reductase and p53 are necessary for lipid homeostasis and tumor suppression through the ABCA1-SREBP pathway
Abstract
p53 is known to modulate metabolism and FDXR is required for steroidogenesis. Given that FDXR is a target/regulator of p53, the FDXR-p53 axis may play a unique role in lipid metabolism. Here, we found that expression of ABCA1, a cholesterol-efflux pump, was suppressed by loss of FDXR and/or p53, leading to activation of master lipogenic regulators SREBP1/2. Accordingly, lipid droplets, cholesterol, and triglycerides were increased by loss of FDXR or p53, which were further increased by loss of both FDXR and p53. To explore the biological significance of the FDXR-p53 axis, we generated a cohort of mice deficient in Fdxr and/or Trp53. We found that Fdxr+/-, Trp53+/-, and Fdxr+/-;Trp53+/- mice had a short life span and were prone to spontaneous tumors and liver steatosis. Moreover, the levels of serum cholesterol and triglycerides were significantly increased in Fdxr+/- and Trp53+/- mice, which were further increased in Fdxr+/-;Trp53+/- mice. Interestingly, loss of Fdxr but not p53 led to accumulation of serum low-density lipoprotein. Together, our findings reveal that the FDXR-p53 axis plays a critical role in lipid homeostasis and tumor suppression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
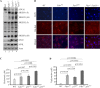
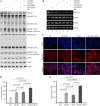
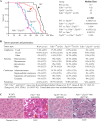
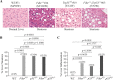
References
-
- Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91. - PubMed
-
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–23. - PubMed
-
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol cell Biol. 2008;9:162–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

