A methodological checklist for fMRI drug cue reactivity studies: development and expert consensus
- PMID: 35121856
- PMCID: PMC9063851
- DOI: 10.1038/s41596-021-00649-4
A methodological checklist for fMRI drug cue reactivity studies: development and expert consensus
Abstract
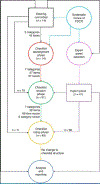
Cue reactivity is one of the most frequently used paradigms in functional magnetic resonance imaging (fMRI) studies of substance use disorders (SUDs). Although there have been promising results elucidating the neurocognitive mechanisms of SUDs and SUD treatments, the interpretability and reproducibility of these studies is limited by incomplete reporting of participants' characteristics, task design, craving assessment, scanning preparation and analysis decisions in fMRI drug cue reactivity (FDCR) experiments. This hampers clinical translation, not least because systematic review and meta-analysis of published work are difficult. This consensus paper and Delphi study aims to outline the important methodological aspects of FDCR research, present structured recommendations for more comprehensive methods reporting and review the FDCR literature to assess the reporting of items that are deemed important. Forty-five FDCR scientists from around the world participated in this study. First, an initial checklist of items deemed important in FDCR studies was developed by several members of the Enhanced NeuroImaging Genetics through Meta-Analyses (ENIGMA) Addiction working group on the basis of a systematic review. Using a modified Delphi consensus method, all experts were asked to comment on, revise or add items to the initial checklist, and then to rate the importance of each item in subsequent rounds. The reporting status of the items in the final checklist was investigated in 108 recently published FDCR studies identified through a systematic review. By the final round, 38 items reached the consensus threshold and were classified under seven major categories: 'Participants' Characteristics', 'General fMRI Information', 'General Task Information', 'Cue Information', 'Craving Assessment Inside Scanner', 'Craving Assessment Outside Scanner' and 'Pre- and Post-Scanning Considerations'. The review of the 108 FDCR papers revealed significant gaps in the reporting of the items considered important by the experts. For instance, whereas items in the 'General fMRI Information' category were reported in 90.5% of the reviewed papers, items in the 'Pre- and Post-Scanning Considerations' category were reported by only 44.7% of reviewed FDCR studies. Considering the notable and sometimes unexpected gaps in the reporting of items deemed to be important by experts in any FDCR study, the protocols could benefit from the adoption of reporting standards. This checklist, a living document to be updated as the field and its methods advance, can help improve experimental design, reporting and the widespread understanding of the FDCR protocols. This checklist can also provide a sample for developing consensus statements for protocols in other areas of task-based fMRI.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures






References
-
- Ekhtiari H. et al. Functional neuroimaging for addiction medicine: from mechanisms to practical considerations. Prog. Brain Res 224, 129–153 (2016). - PubMed
-
- Ekhtiari H. et al. Neuroscience of drug craving for addiction medicine: from circuits to therapies. Prog. Brain Res 223, 115–141 (2016). - PubMed
-
- Ekhtiari H. & ACRI Secretariat. A systematic review on fMRI drug cue reactivity studies (OSF, 2020). https://osf.io/eb972/(2020).
Publication types
MeSH terms
Grants and funding
- R01 AA022328/AA/NIAAA NIH HHS/United States
- U24 DA041147/DA/NIDA NIH HHS/United States
- R01 DA047851/DA/NIDA NIH HHS/United States
- R01 DA048301/DA/NIDA NIH HHS/United States
- R21 DA044465/DA/NIDA NIH HHS/United States
- R01 DA030344/DA/NIDA NIH HHS/United States
- P30 DA048742/DA/NIDA NIH HHS/United States
- P50 AA010761/AA/NIAAA NIH HHS/United States
- K02 DA042987/DA/NIDA NIH HHS/United States
- R01 DA054275/DA/NIDA NIH HHS/United States
- K23 AA023894/AA/NIAAA NIH HHS/United States
- K23 DA042898/DA/NIDA NIH HHS/United States
- R01 DA041528/DA/NIDA NIH HHS/United States
- F32 AA027699/AA/NIAAA NIH HHS/United States
- R01 AT010627/AT/NCCIH NIH HHS/United States
- T32 DA024635/DA/NIDA NIH HHS/United States
- R01 AA026859/AA/NIAAA NIH HHS/United States
- R01 AA026844/AA/NIAAA NIH HHS/United States
- UG1 DA050209/DA/NIDA NIH HHS/United States
- R01 DA039135/DA/NIDA NIH HHS/United States
- R01 AA027765/AA/NIAAA NIH HHS/United States
- K08 AA023545/AA/NIAAA NIH HHS/United States
- R01 AA023665/AA/NIAAA NIH HHS/United States
- U01 AA021692/AA/NIAAA NIH HHS/United States
- R01 DA041438/DA/NIDA NIH HHS/United States
- K12 DA000167/DA/NIDA NIH HHS/United States
- R01 DA053342/DA/NIDA NIH HHS/United States
- R21 HL144673/HL/NHLBI NIH HHS/United States
- U01 DA041089/DA/NIDA NIH HHS/United States
- R21 DA045853/DA/NIDA NIH HHS/United States
- R01 DA040670/DA/NIDA NIH HHS/United States
- R01 DA049547/DA/NIDA NIH HHS/United States
- R01 AA025365/AA/NIAAA NIH HHS/United States
- U24 AA021695/AA/NIAAA NIH HHS/United States
- R01 DA041866/DA/NIDA NIH HHS/United States

