Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression
- PMID: 35122025
- PMCID: PMC9169571
- DOI: 10.1038/s43018-021-00227-3
Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression
Abstract
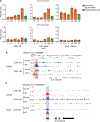
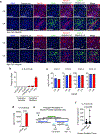
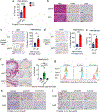
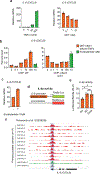
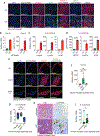
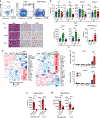
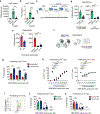
Unlike several other tumor types, prostate cancer rarely responds to immune checkpoint blockade (ICB). To define tumor cell intrinsic factors that contribute to prostate cancer progression and resistance to ICB, we analyzed prostate cancer epithelial cells from castration-sensitive and -resistant samples using implanted tumors, cell lines, transgenic models and human tissue. We found that castration resulted in increased expression of interleukin-8 (IL-8) and its probable murine homolog Cxcl15 in prostate epithelial cells. We showed that these chemokines drove subsequent intratumoral infiltration of tumor-promoting polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), which was largely abrogated when IL-8 signaling was blocked genetically or pharmacologically. Targeting IL-8 signaling in combination with ICB delayed the onset of castration resistance and increased the density of polyfunctional CD8 T cells in tumors. Our findings establish a novel mechanism by which castration mediates IL-8 secretion and subsequent PMN-MDSC infiltration, and highlight blockade of the IL-8/CXCR2 axis as a potential therapeutic intervention.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests
C.G.D. has served as a consultant for Agenus, Dendreon, Janssen Oncology, Eli Lilly, Merck, AstraZeneca, MedImmune, Pierre Fabre, Genentech, and Genocea Biosciences. A.M.C. is a shareholder of Aclaris Therapeutics, Inc, and a consultant for Dermira, Inc. and Aclaris Therapeutics, Inc. Columbia University has filed a US patent claiming the benefit of U.S. Provisional Patent Application No. 62/809,060 (inventors C.G.D. and Z.A.L.B.) on the use of IL-8 / CXCR2 blockade of PMN-MDSC recruitment to the TME for the treatment of prostate cancer. The remaining authors declare no competing interests.
Figures
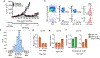



Comment in
-
Behind the IL-8 ball in prostate cancer.Nat Cancer. 2021 Aug;2(8):775-776. doi: 10.1038/s43018-021-00235-3. Nat Cancer. 2021. PMID: 35122026 No abstract available.
References
-
- Kwon ED et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 15, 700–712, doi:10.1016/S1470-2045(14)70189-5 (2014). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

