Seroreduction of syphilis non-treponemal titers during pregnancy for women with and without HIV co-infection
- PMID: 35122676
- PMCID: PMC9516483
- DOI: 10.1002/ijgo.14131
Seroreduction of syphilis non-treponemal titers during pregnancy for women with and without HIV co-infection
Abstract
Objective: To evaluate the effect of HIV co-infection on non-treponemal titers during pregnancy in women with syphilis.
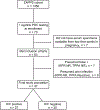
Methods: This is a secondary analysis of pregnant women with syphilis in the prospective, observational Zambian Preterm Birth Prevention Study (ZAPPS). Treponemal (Treponema pallidum particle agglutination) and non-treponemal (rapid plasma reagin; RPR) testing were performed on serum biospecimens, resulting in 47 participants with serologically confirmed syphilis (27 HIV-positive, 20 HIV-negative). The primary outcome, achievement of RPR titer seroreduction during pregnancy, was analyzed by logistic regression. Secondary outcomes included overall titer reduction, seroreduction rate, serologic cure, and adverse pregnancy outcomes.
Results: Seroreduction of RPR titer occurred in 78% (21/27) of women with HIV versus 45% (9/20) of women without (adjusted odds ratio 4.66; 95% confidence interval [CI] 1.14 - 19.08). Overall RPR titer reduction, rate of seroreduction per week, and the proportion achieving serologic cure each trended higher among women with HIV compared with those without HIV. There was a trend toward decreased stillbirth incidence in participants achieving seroreduction (odds ratio 0.15, 95% CI 0.01-1.58).
Conclusion: HIV co-infection in this cohort of Zambian women with syphilis was associated with greater odds of RPR titer seroreduction during pregnancy. Pregnant women with syphilis and HIV may not be at increased risk for a delayed syphilis treatment response compared with women without HIV.
Keywords: Treponema pallidum; HIV; Zambia; pregnancy; rapid plasma reagin; serology; stillbirth; syphilis.
© 2022 International Federation of Gynecology and Obstetrics.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

