Profiling the Bisecting N-acetylglucosamine Modification in Amniotic Membrane via Mass Spectrometry
- PMID: 35123071
- PMCID: PMC9880894
- DOI: 10.1016/j.gpb.2021.09.010
Profiling the Bisecting N-acetylglucosamine Modification in Amniotic Membrane via Mass Spectrometry
Abstract
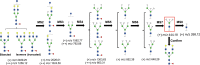
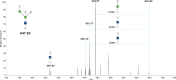
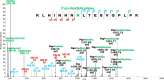
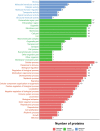
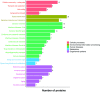
Bisecting N-acetylglucosamine (GlcNAc), a GlcNAc linked to the core β-mannose residue via a β1,4 linkage, is a special type of N-glycosylation that has been reported to be involved in various biological processes, such as cell adhesion and fetal development. This N-glycan structure is abundant in human trophoblasts, which is postulated to be resistant to natural killer cell-mediated cytotoxicity, enabling a mother to nourish a fetus without rejection. In this study, we hypothesized that the human amniotic membrane, which serves as the last barrier for the fetus, may also express bisected-type glycans. To test this hypothesis, glycomic analysis of the human amniotic membrane was performed, and bisected N-glycans were detected. Furthermore, our proteomic data, which have been previously employed to explore human missing proteins, were analyzed and the presence of bisecting GlcNAc-modified peptides was confirmed. A total of 41 glycoproteins with 43 glycopeptides were found to possess a bisecting GlcNAc, and 25 of these glycoproteins were reported to exhibit this type of modification for the first time. These results provide insights into the potential roles of bisecting GlcNAc modification in the human amniotic membrane, and can be beneficial to functional studies on glycoproteins with bisecting GlcNAc modifications and functional studies on immune suppression in human placenta.
Keywords: Amniotic membrane; Bisecting N-acetylglucosamine; Glycan; Glycoprotein; Mass spectrometry.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Kizuka Y., Taniguchi N. Neural functions of bisecting GlcNAc. Glycoconj J. 2018;35:345–351. - PubMed
-
- Schachter H. Complex N-glycans: the story of the “yellow brick road”. Glycoconj J. 2014;31:1–5. - PubMed
-
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem. 1980;255:4894–4902. - PubMed
-
- Yamashita K., Tachibana Y., Kobata A. The structures of the galactose-containing sugar chains of ovalbumin. J Biol Chem. 1978;253:3862–3869. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

