The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione
- PMID: 35123263
- PMCID: PMC8800159
- DOI: 10.1016/j.bbrc.2022.01.106
The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione
Abstract
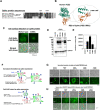
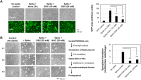
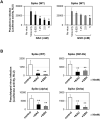
Viral spike proteins play important roles in the viral entry process, facilitating attachment to cellular receptors and fusion of the viral envelope with the cell membrane. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein binds to the cellular receptor angiotensin converting enzyme-2 (ACE2) via its receptor-binding domain (RBD). The cysteine residue at position 488, consisting of a disulfide bridge with cysteine 480 is located in an important structural loop at ACE2-binding surface of RBD, and is highly conserved among SARS-related coronaviruses. We showed that the substitution of Cys-488 with alanine impaired pseudotyped SARS-CoV-2 infection, syncytium formation, and cell-cell fusion triggered by SARS-CoV-2 spike expression. Consistently, in vitro binding of RBD and ACE2, spike-mediated cell-cell fusion, and pseudotyped viral infection of VeroE6/TMPRSS2 cells were inhibited by the thiol-reactive compounds N-acetylcysteine (NAC) and a reduced form of glutathione (GSH). Furthermore, we demonstrated that the activity of variant spikes from the SARS-CoV-2 alpha and delta strains were also suppressed by NAC and GSH. Taken together, these data indicate that Cys-488 in spike RBD is required for SARS-CoV-2 spike functions and infectivity, and could be a target of anti-SARS-CoV-2 therapeutics.
Keywords: Cysteine; SARS-CoV-2; Spike.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

