A randomized, double-blind, placebo-controlled phase II trial to explore the effects of a GABAA-α5 NAM (basmisanil) on intellectual disability associated with Down syndrome
- PMID: 35123401
- PMCID: PMC8903644
- DOI: 10.1186/s11689-022-09418-0
A randomized, double-blind, placebo-controlled phase II trial to explore the effects of a GABAA-α5 NAM (basmisanil) on intellectual disability associated with Down syndrome
Abstract
Background: There are currently no pharmacological therapies to address the intellectual disability associated with Down syndrome. Excitatory/inhibitory imbalance has been hypothesized to contribute to impairments in cognitive functioning in Down syndrome. Negative modulation of the GABAA-α5 receptor is proposed as a mechanism to attenuate GABAergic function and restore the excitatory/inhibitory balance.
Methods: Basmisanil, a selective GABAA-α5 negative allosteric modulator, was evaluated at 120 mg or 240 mg BID (80 or 160 mg for 12-13 years) in a 6-month, randomized, double-blind, placebo-controlled phase II trial (Clematis) for efficacy and safety in adolescents and young adults with Down syndrome. The primary endpoint was based on a composite analysis of working memory (Repeatable Battery for the Assessment of Neuropsychological Scale [RBANS]) and independent functioning and adaptive behavior (Vineland Adaptive Behavior Scales [VABS-II] or the Clinical Global Impression-Improvement [CGI-I]). Secondary measures included the Behavior Rating Inventory of Executive Functioning-Preschool (BRIEF-P), Clinical Evaluation of Language Fundamentals (CELF-4), and Pediatric Quality of Life Inventory (Peds-QL). EEG was conducted for safety monitoring and quantitatively analyzed in adolescents.
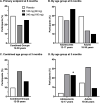
Results: Basmisanil was safe and well-tolerated; the frequency and nature of adverse events were similar in basmisanil and placebo arms. EEG revealed treatment-related changes in spectral power (increase in low ~ 4-Hz and decrease in high ~ 20-Hz frequencies) providing evidence of functional target engagement. All treatment arms had a similar proportion of participants showing above-threshold improvement on the primary composite endpoint, evaluating concomitant responses in cognition and independent functioning (29% in placebo, 20% in low dose, and 25% in high dose). Further analysis of the individual measures contributing to the primary endpoint revealed no difference between placebo and basmisanil-treated groups in either adolescents or adults. There were also no differences across the secondary endpoints assessing changes in executive function, language, or quality of life.
Conclusions: Basmisanil did not meet the primary efficacy objective of concomitant improvement on cognition and adaptive functioning after 6 months of treatment, despite evidence for target engagement. This study provides key learnings for future clinical trials in Down syndrome.
Trial registration: The study was registered on December 31, 2013, at clinicaltrials.gov as NCT02024789.
Keywords: Adaptive behavior; Cognition; Down syndrome; EEG; GABAA-α5.
© 2022. The Author(s).
Conflict of interest statement
At the time of the study, P Fontoura, C Goeldner, MC Hernandez, JF Hipp, O Khwaja, X Liogier d’Ardhuy, J Noeldeke, S Pellicer, L Squassante, C Wandel were employees of F.Hoffmann-La Roche AG Switzerland; M Derks and S Lennon-Chrimes were employees of Roche Products Ltd. UK; J Visootsak was an employee of Roche New York. All employees (former and current) may be eligible for stock and stock options. P S Kishnani has no disclosures for Down syndrome-related research. J Lirio Casero has no disclosures. B G Skotko occasionally consults on the topic of Down syndrome through the Gerson Lehrman Group. He receives remuneration from Down syndrome non-profit organizations for speaking engagements and associated travel expenses. Dr. Skotko receives annual royalties from Woodbine House, Inc., for the publication of his book,
Figures


References
-
- de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017;19(4):439–447. - PubMed
-
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74(1):75–93. - PubMed
-
- Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet. 2015;169(2):135–149. - PubMed
-
- Golden JA, Hyman BT. Development of the superior temporal neocortex is anomalous in trisomy 21. J Neuropathol Exp Neurol. 1994;53(5):513–520. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

