Long-term efficacy and safety of SARS-CoV-2 vaccination in patients with chronic kidney disease, on dialysis or after kidney transplantation: a national prospective observational cohort study
- PMID: 35123437
- PMCID: PMC8817171
- DOI: 10.1186/s12882-022-02680-3
Long-term efficacy and safety of SARS-CoV-2 vaccination in patients with chronic kidney disease, on dialysis or after kidney transplantation: a national prospective observational cohort study
Abstract
Background: COVID-19 is associated with increased morbidity and mortality in patients with chronic kidney disease (CKD) stages G4-G5, on dialysis or after kidney transplantation (kidney replacement therapy, KRT). SARS-CoV-2 vaccine trials do not elucidate if SARS-CoV-2 vaccination is effective in these patients. Vaccination against other viruses is known to be less effective in kidney patients. Our objective is to assess the efficacy and safety of various types of SARS-CoV-2 vaccinations in patients with CKD stages G4-G5 or on KRT.
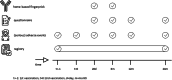
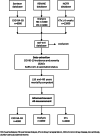
Methods: In this national prospective observational cohort study we will follow patients with CKD stages G4-G5 or on KRT (n = 12,000) after SARS-CoV-2 vaccination according to the Dutch vaccination program. Blood will be drawn for antibody response measurements at day 28 and month 6 after completion of vaccination. Patient characteristics and outcomes will be extracted from registration data and questionnaires during 2 years of follow-up. Results will be compared with a control group of non-vaccinated patients. The level of antibody response to vaccination will be assessed in subgroups to predict protection against COVID-19 breakthrough infection.
Results: The primary endpoint is efficacy of SARS-CoV-2 vaccination determined as the incidence of COVID-19 after vaccination. Secondary endpoints are the antibody based immune response at 28 days after vaccination, the durability of this response at 6 months after vaccination, mortality and (serious) adverse events.
Conclusion: This study will fulfil the lack of knowledge on efficacy and safety of SARS-CoV-2 vaccination in patients with CKD stages G4-G5 or on KRT.
Trial registration: The study protocol has been registered in clinicaltrials.gov ( NCT04841785 ). Current knowledge about this subject COVID-19 has devastating impact on patients with CKD stages G4-G5, on dialysis or after kidney transplantation. Effective SARS-CoV-2 vaccination is very important in these vulnerable patient groups. Recent studies on vaccination in these patient groups are small short-term studies with surrogate endpoints. Contribution of this study Assessment of incidence and course of COVID-19 after various types of SARS-CoV-2 vaccination during a two-year follow-up period in not only patients on dialysis or kidney transplant recipients, but also in patients with CKD stages G4-G5. Quantitative analysis of antibody response after SARS-CoV-2 vaccination and its relationship with incidence and course of COVID-19 in patients with CKD stages G4-G5, on dialysis or after kidney transplantation compared with a control group. Monitoring of (serious) adverse events and development of anti-HLA antibodies. Impact on practice or policy Publication of the study design contributes to harmonization of SARS-CoV-2 vaccine study methodology in kidney patients at high-risk for severe COVID-19. Data on efficacy of SARS-CoV-2 vaccination in patients with CKD will provide guidance for future vaccination policy.
Keywords: Antibody response; Chronic kidney disease; Dialysis; Efficacy; Kidney transplantation; SARS-CoV-2; Safety; Vaccine.
© 2022. The Author(s).
Conflict of interest statement
None declared.
Figures
References
-
- Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

