Serologic response following SARS-COV2 vaccination in patients with cancer: a systematic review and meta-analysis
- PMID: 35123511
- PMCID: PMC8817639
- DOI: 10.1186/s13045-022-01233-3
Serologic response following SARS-COV2 vaccination in patients with cancer: a systematic review and meta-analysis
Abstract
Purpose: Patients with cancer have an increased risk of coronavirus disease 2019 (COVID-19) and an attenuated responses to various vaccines. This meta-analysis aims to assess the serologic response to COVID-19 vaccination in patients with cancer.
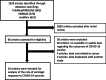
Methods: Electronic databases were systematically searched on August 1, 2021 for studies that reported the serologic response to COVID-19 vaccine in cancer patients. Random effects models were used to achieve pooled serologic response rates and odds ratios (ORs).
Results: We analyzed 16 observational studies with a total of 1453 patients with cancer. A majority of studies used mRNA vaccines (BNT162b2 or mRNA-1273). The proportion of patients achieving a serologic response after a single and two doses of COVID-19 vaccine were 54.2% (95% confidence interval [CI] 41.0-66.9) and 87.7% (95% CI 82.5-91.5), respectively. Patients with hematologic cancers had a lower response rate after the second dose of vaccine compared to those with solid organ cancers (63.7% vs. 94.9%), which was attributable to the low response rates associated with certain conditions (chronic lymphocytic leukemia, lymphoma) and therapies (anti-CD20, kinase inhibitors). A lower proportion of patients with cancer achieved a serologic response compared to control patients after one and two doses of vaccine (OR0.073 [95% CI 0.026-0.20] and 0.10 [95% CI 0.039-0.26], respectively).
Conclusions: Patients with cancer, especially those with hematologic B-cell malignancies, have a lower serologic response to COVID-19 vaccines. The results suggest that cancer patients should continue to follow safety measures including mask-wearing after vaccination and suggest the need for additional strategies for prophylaxis.
Keywords: COVID-19; Cancer; Immunocompromised; Meta-analysis; Outcomes; Vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. - DOI - PMC - PubMed
-
- Statistics NCfH. 2019 2021.9.10. National Health Interview Survey. https://www.cdc.gov/nchs/nhis/about_nhis.htm. 2021.9.10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

