The hallmarks of cancer metabolism: Still emerging
- PMID: 35123658
- PMCID: PMC8891094
- DOI: 10.1016/j.cmet.2022.01.007
The hallmarks of cancer metabolism: Still emerging
Abstract
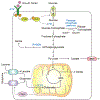
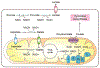
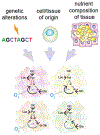
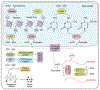
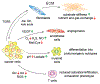
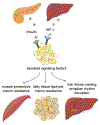
Metabolism of cancer cells is geared toward biomass production and proliferation. Since the metabolic resources within the local tissue are finite, this can lead to nutrient depletion and accumulation of metabolic waste. To maintain growth in these conditions, cancer cells employ a variety of metabolic adaptations, the nature of which is collectively determined by the physiology of their cell of origin, the identity of transforming lesions, and the tissue in which cancer cells reside. Furthermore, select metabolites not only serve as substrates for energy and biomass generation, but can also regulate gene and protein expression and influence the behavior of non-transformed cells in the tumor vicinity. As they grow and metastasize, tumors can also affect and be affected by the nutrient distribution within the body. In this hallmark update, recent advances are incorporated into a conceptual framework that may help guide further research efforts in exploring cancer cell metabolism.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.B.T. is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. He is also a former member of the Board of Directors and stockholder of Merck and Charles River Laboratories. He holds patents related to cellular metabolism. He is a member of the advisory board of Cell Metabolism. N.N.P. and J.Z. declare no competing interests.
Figures

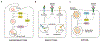


References
-
- Andrzejewski S, Klimcakova E, Johnson RM, Tabaries S, Annis MG, McGuirk S, Northey JJ, Chenard V, Sriram U, Papadopoli DJ, et al. (2017). PGC-1alpha Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab 26, 778–787 e775 - PubMed
-
- Avissar NE, Sax HC, and Toia L (2008). In human entrocytes, GLN transport and ASCT2 surface expression induced by short-term EGF are MAPK, PI3K, and Rho-dependent. Dig Dis Sci 53, 2113–2125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

