COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study
- PMID: 35123676
- PMCID: PMC8813209
- DOI: 10.1016/S2468-1253(22)00005-X
COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study
Abstract
Background: The effects that therapies for inflammatory bowel disease (IBD) have on immune responses to SARS-CoV-2 vaccination are not yet fully known. Therefore, we sought to determine whether COVID-19 vaccine-induced antibody responses were altered in patients with IBD on commonly used immunosuppressive drugs.
Methods: In this multicentre, prospective, case-control study (VIP), we recruited adults with IBD treated with one of six different immunosuppressive treatment regimens (thiopurines, infliximab, a thiopurine plus infliximab, ustekinumab, vedolizumab, or tofacitinib) and healthy control participants from nine centres in the UK. Eligible participants were aged 18 years or older and had received two doses of COVID-19 vaccines (either ChAdOx1 nCoV-19 [Oxford-AstraZeneca], BNT162b2 [Pfizer-BioNTech], or mRNA1273 [Moderna]) 6-12 weeks apart (according to scheduling adopted in the UK). We measured antibody responses 53-92 days after a second vaccine dose using the Roche Elecsys Anti-SARS-CoV-2 spike electrochemiluminescence immunoassay. The primary outcome was anti-SARS-CoV-2 spike protein antibody concentrations in participants without previous SARS-CoV-2 infection, adjusted by age and vaccine type, and was analysed by use of multivariable linear regression models. This study is registered in the ISRCTN Registry, ISRCTN13495664, and is ongoing.
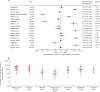
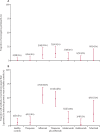
Findings: Between May 31 and Nov 24, 2021, we recruited 483 participants, including patients with IBD being treated with thiopurines (n=78), infliximab (n=63), a thiopurine plus infliximab (n=72), ustekinumab (n=57), vedolizumab (n=62), or tofacitinib (n=30), and 121 healthy controls. We included 370 participants without evidence of previous infection in our primary analysis. Geometric mean anti-SARS-CoV-2 spike protein antibody concentrations were significantly lower in patients treated with infliximab (156·8 U/mL [geometric SD 5·7]; p<0·0001), infliximab plus thiopurine (111·1 U/mL [5·7]; p<0·0001), or tofacitinib (429·5 U/mL [3·1]; p=0·0012) compared with controls (1578·3 U/mL [3·7]). There were no significant differences in antibody concentrations between patients treated with thiopurine monotherapy (1019·8 U/mL [4·3]; p=0·74), ustekinumab (582·4 U/mL [4·6]; p=0·11), or vedolizumab (954·0 U/mL [4·1]; p=0·50) and healthy controls. In multivariable modelling, lower anti-SARS-CoV-2 spike protein antibody concentrations were independently associated with infliximab (geometric mean ratio 0·12, 95% CI 0·08-0·17; p<0·0001) and tofacitinib (0·43, 0·23-0·81; p=0·0095), but not with ustekinumab (0·69, 0·41-1·19; p=0·18), thiopurines (0·89, 0·64-1·24; p=0·50), or vedolizumab (1·16, 0·74-1·83; p=0·51). mRNA vaccines (3·68, 2·80-4·84; p<0·0001; vs adenovirus vector vaccines) were independently associated with higher antibody concentrations and older age per decade (0·79, 0·72-0·87; p<0·0001) with lower antibody concentrations.
Interpretation: For patients with IBD, the immunogenicity of COVID-19 vaccines varies according to immunosuppressive drug exposure, and is attenuated in recipients of infliximab, infliximab plus thiopurines, and tofacitinib. Scheduling of third primary, or booster, doses could be personalised on the basis of an individual's treatment, and patients taking anti-tumour necrosis factor and tofacitinib should be prioritised.
Funding: Pfizer.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JLA reports sponsorship from Vifor Pharma for accommodation and travel to the British Society of Gastroenterology annual meeting 2019, outside the submitted work. NAK reports grants from AbbVie, Biogen, Celgene, Celtrion, Galapagos, MSD, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; consulting fees from Amgen, Bristol Myers Squibb, Falk, Janssen, Mylan, Pharmacosmos, Galapagos, Takeda, and Tillotts; personal fees from Allergan, Celltrion, Falk, Ferring, Janssen, Pharmacosmos, Takeda, Tilllotts, and Galapagos; and support for attending meetings from AbbVie, Falk, and Janssen, outside the submitted work. AS has received travel expense support from Janssen. SS reports grants from Takeda, AbbVie, Tillots Pharma, Janssen, Pfizer, and Biogen, and personal fees from Takeda, AbbVie, Janssen, Pharmacocosmos, Biogen, Pfizer, Tillots Pharma, and Falk Pharma, outside the submitted work. ALH reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, AZ, Atlantic, Bristol Myers Squibb, Celltrion, Falk, Galapogos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire, and Takeda; participation on the Global Steering Committee for Genentech; support for attending meetings from AbbVie, Takeda, and Janssen; and participation on a data safety monitoring board or advisory board for AbbVie, AZ, Atlantic, Bristol Myers Squibb, Galapogos, Janssen, Pfizer, and Takeda. PMI reports grants from Celltrion, Takeda, MSD, Pfizer, and Galapagos, and personal fees from Celltrion, Takeda, Pfizer, Galapagos, Gilead, AbbVie, Janssen, Bristol Myers Squibb, Lilly, and Arena, outside the submitted work. MP receives unrestricted educational grants from Pfizer for genetic analyses to support the IBD BioResource and speaker fees from Janssen. GRJ has received grants from the Wellcome Trust and ECCO; speaker fees from Takeda, Ferring, and Janssen; and support for attending meetings or travel from Ferring. KK reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen and Ferring; support for attending meetings or travel from Janssen and Takeda; and participation on a data safety monitoring board or advisory board for Janssen and PredictImmune. KVP reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, DrFalk, Janssen, PreddictImmune, and Takeda; support for attending meetings or travel from AbbVie, Ferring, Janssen, and Tillots; and participation on a data safety monitoring board or advisory board for AbbVie, Galapagos, and Janssen. AJK reports consulting fees from Janssen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer and Takeda; support for attending meetings or travel from Janssen, Tillots, and Norgine; and participation on a data safety monitoring board or advisory board for AbbVie. LCH reports support for attending meetings or travel from AbbVie. CWL reports a Future Leaders Fellow award from UK Research and Innovation; personal consulting fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, and Iterative Scopes; institutional consulting fees from Trellus Health; personal fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresnius Kabi, Ferring, and Dr Falk; and support for attending meetings from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresnius Kabi, Ferring, and Dr Falk. RJB and DMA are members of the Global T cell Expert Consortium and have consulted for Oxford Immunotec outside the submitted work. JRG reports grants from F Hoffmann-La Roche, Biogen, Celltrion Healthcare, and Galapagos, and non-financial support from Immundiagnostik, during the conduct of the study. TA reports grant funding from Pfizer to his institution to deliver this study; grants from Celltrion, Roche, Takeda, Biogen, and Galapagos; and honoraria for lectures from Takeda and Roche, outside the submitted work. NP is the principal investigator on the research grant from Pfizer that helped to fund the VIP study; has received research grants from Bristol Myers Squibb outside the submitted work; reports personal fees from Takeda, Janssen, Pfizer, Bristol Myers Squibb, AbbVie, Roche, Lilly, Allergan, and Celgene, outside the submitted work; and has served as a speaker or advisory board member for AbbVie, Allergan, Bristol Myers Squibb, Celgene, Falk, Ferring, Janssen, Pfizer, Tillotts, Takeda, and Vifor Pharma. All other authors declare no competing interests.
Figures

Comment in
-
Is the attenuated humoral response to COVID-19 vaccination in anti-TNF users relevant?Lancet Gastroenterol Hepatol. 2022 Apr;7(4):280-282. doi: 10.1016/S2468-1253(22)00040-1. Epub 2022 Feb 4. Lancet Gastroenterol Hepatol. 2022. PMID: 35123675 Free PMC article. No abstract available.
References
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. - PubMed
-
- Pratt PK, Jr, David N, Weber HC, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24:380–386. - PubMed
-
- Fiorino G, Peyrin-Biroulet L, Naccarato P, et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18:1042–1047. - PubMed
-
- Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

