A comprehensive assessment of long-term SARS-CoV-2-specific adaptive immune memory in convalescent COVID-19 Solid Organ Transplant recipients
- PMID: 35124011
- PMCID: PMC8813192
- DOI: 10.1016/j.kint.2021.12.029
A comprehensive assessment of long-term SARS-CoV-2-specific adaptive immune memory in convalescent COVID-19 Solid Organ Transplant recipients
Abstract
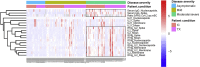
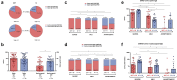
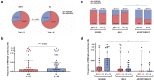
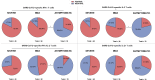
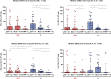
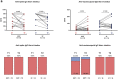
Long-term adaptive immune memory has been reported among immunocompetent individuals up to eight months following SARS-CoV-2 infection. However, limited data is available in convalescent patients with a solid organ transplant. To investigate this, we performed a thorough evaluation of adaptive immune memory at different compartments (serological, memory B cells and cytokine [IFN-γ, IL-2, IFN-γ/IL12 and IL-21] producing T cells) specific to SARS-CoV-2 by ELISA and FluoroSpot-based assays in 102 convalescent patients (53 with a solid organ transplants (38 kidney, 5 liver, 5 lung and 5 heart transplant) and 49 immunocompetent controls) with different clinical COVID-19 severity (severe, mild and asymptomatic) beyond six months after infection. While similar detectable memory responses at different immune compartments were detected between those with a solid organ transplant and immunocompetent individuals, these responses were predominantly driven by distinct COVID-19 clinical severities (97.6%, 80.5% and 42.1%, all significantly different, were seropositive; 84% vs 75% vs 35.7%, all significantly different, showed IgG-producing memory B cells and 82.5%, 86.9% and 31.6%, displayed IFN-γ producing T cells; in severe, mild and asymptomatic convalescent patients, respectively). Notably, patients with a solid organ transplant with longer time after transplantation did more likely show detectable long-lasting immune memory, regardless of COVID-19 severity. Thus, our study shows that patients with a solid organ transplant are capable of maintaining long-lasting peripheral immune memory after COVID-19 infection; mainly determined by the degree of infection severity.
Keywords: COVID-19 infection; adaptive immunity; solid organ transplantation.
Copyright © 2022 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;2019:3–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

