Contribution of the caudal medullary raphe to opioid induced respiratory depression
- PMID: 35124284
- PMCID: PMC8897277
- DOI: 10.1016/j.resp.2022.103855
Contribution of the caudal medullary raphe to opioid induced respiratory depression
Abstract
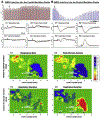
Background: Opioid-induced respiratory depression can be partially antagonized in the preBötzinger Complex and Parabrachial Nucleus/Kölliker-Fuse Complex. We hypothesized that additional opioid antagonism in the caudal medullary raphe completely reverses the opioid effect.
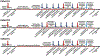
Methods: In adult ventilated, vagotomized, decerebrate rabbits, we administrated remifentanil intravenously at "analgesic", "apneic", and "very high" doses and determined the reversal with sequential naloxone microinjections into the bilateral Parabrachial Nucleus/Kölliker-Fuse Complex, preBötzinger Complex, and caudal medullary raphe. In separate animals, we injected opioid antagonists into the raphe without intravenous remifentanil.
Results: Sequential naloxone microinjections completely reversed respiratory rate depression from "analgesic" and "apneic" remifentanil, but not "very high" remifentanil concentrations. Antagonist injection into the caudal medullary raphe without remifentanil independently increased respiratory rate.
Conclusions: Opioid-induced respiratory depression results from a combined effect on the respiratory rhythm generator and respiratory drive. The effect in the caudal medullary raphe is complex as we also observed local antagonism of endogenous opioid receptor activation, which has not been described before.
Keywords: Caudal medullary raphe; Opioid-induced respiratory depression; Parabrachial Nucleus/ kölliker-fuse complex; Respiratory phase timing; Respiratory rhythm generator; preBötzinger complex.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

