Chalcones from Angelica keiskei (ashitaba) inhibit key Zika virus replication proteins
- PMID: 35124513
- PMCID: PMC9187613
- DOI: 10.1016/j.bioorg.2022.105649
Chalcones from Angelica keiskei (ashitaba) inhibit key Zika virus replication proteins
Abstract
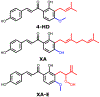
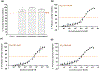
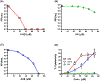
Zika virus (ZIKV) is a dangerous human pathogen and no antiviral drugs have been approved to date. The chalcones are a group of small molecules that are found in a number of different plants, including Angelica keiskei Koidzumi, also known as ashitaba. To examine chalcone anti-ZIKV activity, three chalcones, 4-hydroxyderricin (4HD), xanthoangelol (XA), and xanthoangelol-E (XA-E), were purified from a methanol-ethyl acetate extract from A. keiskei. Molecular and ensemble docking predicted that these chalcones would establish multiple interactions with residues in the catalytic and allosteric sites of ZIKV NS2B-NS3 protease, and in the allosteric site of the NS5 RNA-dependent RNA-polymerase (RdRp). Machine learning models also predicted 4HD, XA and XA-E as potential anti-ZIKV inhibitors. Enzymatic and kinetic assays confirmed chalcone inhibition of the ZIKV NS2B-NS3 protease allosteric site with IC50s from 18 to 50 µM. Activity assays also revealed that XA, but not 4HD or XA-E, inhibited the allosteric site of the RdRp, with an IC50 of 6.9 µM. Finally, we tested these chalcones for their anti-viral activity in vitro with Vero cells. 4HD and XA-E displayed anti-ZIKV activity with EC50 values of 6.6 and 22.0 µM, respectively, while XA displayed relatively weak anti-ZIKV activity with whole cells. With their simple structures and relative ease of modification, the chalcones represent attractive candidates for hit-to-lead optimization in the search of new anti-ZIKV therapeutics.
Keywords: Angelica keiskei; Ashitaba; Chalcones; Polymerase; Protease; Zika virus.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
S.E. is owner and K.M.Z and D.H.F. work for Collaborations Pharmaceuticals, Inc. Other authors declare no competing financial interest.
Figures
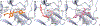
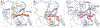
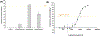

References
-
- Shiryaev SA, Farhy C, Pinto A, Huang CT, Simonetti N, Ngono AE, Dewing A, Shresta S, Pinkerton AB, Cieplak P, Strongin AY, Terskikh AV, Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists, Antiviral Res 143 (2017) 218–229. 10.1016/j.antiviral.2017.04.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

